1-Naphthyl acetonitrile
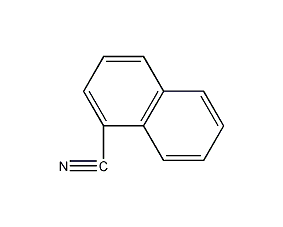

Structural formula
| Business number | 03NR |
|---|---|
| Molecular formula | C12H9N |
| Molecular weight | 167.21 |
| label |
1-Naphthyl acetonitrile, 1-Naphthyl acetonitrile, aromatic compounds |
Numbering system
CAS number:132-75-2
MDL number:MFCD00004041
EINECS number:205-078-1
RTECS number:AM1240000
BRN number:1101012
PubChem number:24897696
Physical property data
None yet
Toxicological data
Acute toxicity data:
Mouse veinLD50: 180mg/kg
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1、 Molar refractive index:48.87
2、 Molar volume(m3/mol ): 134.4
3、 Isotonic specific volume(90.2K):357.9
4、 Surface tension(3.0 dyne/cm):50.2
5、 Polarizability0.5 10-24 cm3):19.37
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 23.8
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 212
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
basic properties
White crystal. Melting point 32-33℃, boiling point 162-164℃, 191-194℃ (2.4kPa), refractive index (nD20) 1.6192. Insoluble in water.
Storage method
None yet
Synthesis method
Brief description of production methods
The mixture of 695g 1-(chloromethyl)naphthalene, 1500ml methanol, 475ml water, and 350g sodium cyanide was boiled and refluxed, and the reaction was stirred for 2 hours. Steam out 1,300 ml of methanol, cool, and wash the reactant with water until neutral to obtain 670-680 g of 1-naphthalene acetonitrile.
Purpose
Purpose
Organic synthesis intermediates.
-US style=”FONT-SIZE: 9pt; FONT-FAMILY: Arial; mso-fareast-font-family: Arial; mso-font-kerning: 0pt”>10-24cm3):19.37
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 23.8
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 212
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
basic properties
White crystal. Melting point 32-33℃, boiling point 162-164℃, 191-194℃ (2.4kPa), refractive index (nD20) 1.6192. Insoluble in water.
Storage method
None yet
Synthesis method
Brief description of production methods
The mixture of 695g 1-(chloromethyl)naphthalene, 1500ml methanol, 475ml water, and 350g sodium cyanide was boiled and refluxed, and the reaction was stirred for 2 hours. Steam out 1,300 ml of methanol, cool, and wash the reactant with water until neutral to obtain 670-680 g of 1-naphthalene acetonitrile.
Purpose
Purpose
Organic synthesis intermediates.