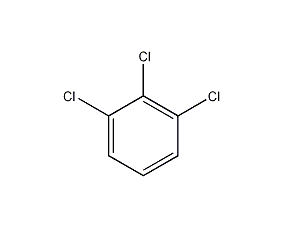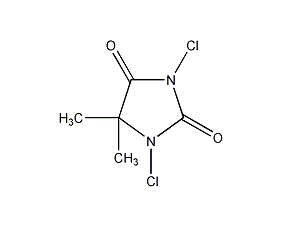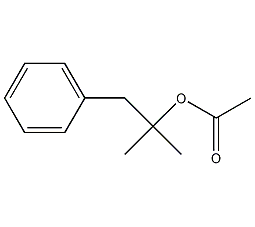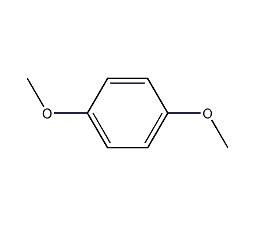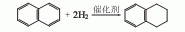
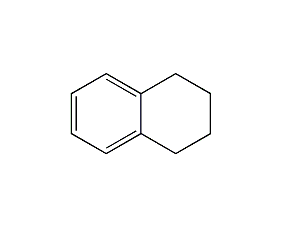
Structural formula
| Business number |
03B5 |
| Molecular formula |
C10H12 |
| Molecular weight |
132 |
| label |
tetralin,
Tetralin,
Tetranap,
aromatic compounds
|
Numbering system
CAS number:119-64-2
MDL number:MFCD00001733
EINECS number:204-340-2
RTECS number:QK3850000
BRN number:1446407
PubChem number:24874115
Physical property data
1. Properties: colorless or light yellow transparent liquid with a menthol-like odor.
2. Boiling point (ºC, 101.3kPa): 207.65
3. Melting point (ºC): -35.75
4. Relative density (g/mL, 20/4ºC): 0.9695
5. Relative density (g/mL, 25/4ºC): 0.9660
6. Refractive index (n20ºC): 1.5414
7. Refractive index (n25ºC): 1.5392
8. Viscosity (mPa·s, 20ºC): 2.02
9. Viscosity (mPa·s, 50ºC): 1.3
p>
10. Flash point (ºC, open): 75
11. Flash point (ºC, closed): 71.6
12. Fire point (ºC): 384
13. Heat of evaporation (KJ/mol, 206ºC): 45.6
14. Heat of generation (KJ/mol): -40.6
15. Heat of combustion ( KJ/(kg·K), constant volume): 5613.2
16. Specific heat capacity (KJ/(kg·K), 15~18ºC): 1.69
17. Critical temperature ( ºC): 417.5
18. Boiling point rising constant: 5.582
19. Thermal conductivity (W/(m·K), 20≤t≤90 ºC): (153.1~ 0.22t)×10-3
20. Explosion lower limit (%, V/V, 77.8ºC): 0.8
21. Explosion upper limit (% , V/V, 121.8ºC): 5.0
22. Volume expansion coefficient (K-1, 10~30ºC): 0.00083
23. Dissolution Properties: Insoluble in water, miscible with most organic solvents such as ethanol, butanol, acetone, benzene, ether, chloroform, petroleum ether, decalin, etc.
24. Critical pressure (MPa): 3.65
25. Critical density (g·cm-3): 0.324
26. Critical volume (cm3·mol-1): 408
27. Critical compression factor: 0.249
28 .Eccentricity factor: 0.328
29. Solubility parameter (J·cm-3)0.5: 19.524
30. van der Waals area (cm2·mol-1): 1.912×109
31. van der Waals Volume (cm3·mol-1): 80.890
32. Gas phase standard combustion heat (enthalpy) (kJ·mol-1 ): -5676.77
33. The gas phase standard claims heat (enthalpy) (kJ·mol-1): 26.65
34.�� Phase standard entropy (J·mol-1·K-1): 366.25
35. Gas phase standard formation free energy (kJ·mol-1): 167.0
36. Gas phase standard hot melt (J·mol-1·K-1): 150.94
37. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -5621.54
38. Liquid phase standard claimed heat ( Enthalpy) (kJ·mol-1): -28.58
39. Liquid phase standard entropy (J·mol-1·K -1): 251.46
40. Liquid phase standard free energy of formation (kJ·mol-1): 147.03
41. Liquid Phase standard hot melt (J·mol-1·K-1): 234.7
Toxicological data
1. Irritation: Rabbit transdermal: 500mg Severe irritation
2. Acute toxicity: Rat oral LD50: 1620ul/kg
Rabbit transdermal LD50: 17300ul/ KG
Guinea pig inhaled LC50: 275ppm3, which is low toxic. It has anesthetic and local stimulating effects. Inhaling the vapor can cause mild confusion, headache, nausea, vomiting, and coughing, and can irritate the conjunctiva and nasal and pharyngeal mucosa or cause rhinitis. Inhalation of high concentrations can damage the liver and kidneys, and cause chemical pneumonitis and crystal opacity. Skin contact may cause dermatitis. The olfactory threshold concentration is 0.018mg/L. TJ 36-79 stipulates that the maximum allowable concentration in workshop air is 100mg/m3. The oral LD50 in rats is 2.9g/kg.
Ecological data
Harmful to water, harmful to fish and plankton.
Molecular structure data
1. Molar refractive index: 43.03
2. Molar volume (cm3/mol): 136.2
3. Isotonic specific volume (90.2K ): 333.5
4. Surface tension (dyne/cm): 35.8
5. Polarizability (10-24cm3): 17.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 92.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, heat, air, and light. It is a flammable liquid and is non-corrosive to metals. Due to the danger of explosion due to the production of peroxide, it should not be evaporated to dryness during distillation. Insoluble in water, miscible with most organic solvents such as ethanol, butanol, acetone, benzene, ether, chloroform, petroleum ether, decalin and so on. Dissolves 50.6% in methanol. In addition to hard glue, shellac, phenolic resin, and urea-formaldehyde resin, tetralin can dissolve most natural and synthetic resins such as rosin, coumarone resin, and alkyd resin. It can also dissolve asphalt, grease, vulcanized rubber, grease and cellulose ethers, but not cellulose esters. 2. Halogenation and nitration reactions can occur to generate halogen and nitro derivatives. After oxidation by air or oxygen, peroxide is generated and gradually turns into a resinous substance. Oxidation with potassium permanganate or nitric acid produces phthalic acid. Phthalic anhydride is generated during gas phase oxidation using a vanadium catalyst. Under nickel catalysis, hydrogenation produces decalin, and dehydrogenation produces naphthalene. Aromatic hydrocarbons such as toluene and xylene are generated during thermal cracking. It reacts with alcohols and alkenes to produce an alkylation reaction on the benzene ring. When in contact with air for a long time, it absorbs oxygen in the air and generates hydroperoxide, which may cause explosion hazard. 3.It is not very toxic, has a certain anesthetic effect, and can cause headaches, discomfort and skin eczema. Rat oral administration: LD502900mg/g. The allowable concentration in air is 0.05 mg/kg.
4. Prolonged contact between tetralin and air can produce tetralin peroxide and cause an explosion. Goggles, gas masks and rubber gloves must be worn during use. 5. Exists in oriental tobacco leaves and smoke.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and water. Keep away from light. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Store in a sealed container. Can be stored in iron, mild steel, copper or aluminum containers.
Tetralin is a Class III flammable liquid and poses a fire hazard. It must be stored in a cool, ventilated place, away from heat sources and fires, and stored separately from oxidants. Pack and unload with care to keep the packaging intact.
Synthesis method
1. Prepared from naphthalene through nickel-catalyzed selective hydrogenation. The gas phase catalytic reaction is carried out in an adiabatic reaction device, using a nickel molybdate catalyst at 300°C and 2.94MPa. The feeding space speed is 0.8h-1, the volume ratio of hydrogen and naphthalene is 2500-6400, and the single-pass conversion rate of naphthalene can reach 85 %, and the selectivity of tetralin is over 92%. After stripping and distillation of the hydrogenated product, the purity of tetralin reaches over 98%.
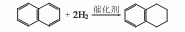
2. Tobacco: OR, 57 .
3. Synthesis: Extraction with pentane.
Purpose
Manadienol is used in large quantities as an intermediate in the manufacture of the insecticide divinyl. It is also used in the production of lubricants to reduce the viscosity of high-viscosity oils. It is widely used as a solvent for resins, waxes, greases, paints, and plastics to remove naphthalene deposits in gas industry equipment. Mixed with alcohol and benzene as fuel for internal combustion engines. It is also used as a degreasing agent, softener, absorbent for low-boiling organic compound vapors, insect repellent and turpentine substitute. Used as solvents for coatings, varnishes, metal soaps, chlorinated rubber, waxes, greases, resins, etc., ink detergents, liquid desiccants and the manufacture of reclaimed rubber.
It can be mixed with all organic solvents except water. It is a solvent with a wide range of dissolution, especially for oils and greases. Therefore, it is used in cotton, rayon, and wool in the fiber industry. Degreasing and washing. In addition, it has universal solubility in resin and can be used as a detergent to remove resin dirt.
The purity of naphthalene is over 98%.
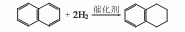
2. Tobacco: OR, 57 .
3. Synthesis: Extraction with pentane.
Purpose
Manadienol is used in large quantities as an intermediate in the manufacture of the insecticide divinyl. It is also used in the production of lubricants to reduce the viscosity of high-viscosity oils. It is widely used as a solvent for resins, waxes, greases, paints, and plastics to remove naphthalene deposits in gas industry equipment. Mixed with alcohol and benzene as fuel for internal combustion engines. It is also used as a degreasing agent, softener, absorbent for low-boiling organic compound vapors, insect repellent and turpentine substitute. Used as solvents for coatings, varnishes, metal soaps, chlorinated rubber, waxes, greases, resins, etc., ink detergents, liquid desiccants and the manufacture of reclaimed rubber.
It can be mixed with all organic solvents except water. It is a solvent with a wide range of dissolution, especially for oils and greases. Therefore, it is used in cotton, rayon, and wool in the fiber industry. Degreasing and washing. In addition, it has universal solubility in resin and can be used as a detergent to remove resin dirt.


![]()