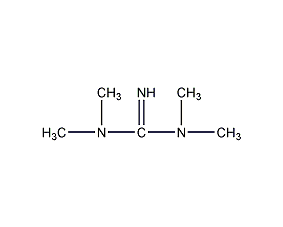
Structural formula
| Business number |
02LL |
| Molecular formula |
C13H13N3 |
| Molecular weight |
211.26 |
| label |
diphenylguanidine,
Rubber vulcanization accelerator DPG,
1,3-diphenylguanidine,
N,N’-diphenylguanidine,
Diphenyl guanidine,
rubber vulcanization accelerator DPG,
1,3-diphenyl guanidine,
N, N’-diphenyl guanidine,
accelerator,
Catalysts and auxiliaries
|
Numbering system
CAS number:102-06-7
MDL number:MFCD00001758
EINECS number:203-002-1
RTECS number:MF0875000
BRN number:1875653
PubChem number:24866918
Physical property data
1. Properties: White powder, odorless, bitter taste.
2. Relative density (g/mL, 25℃): 1.13
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 114~150, 170 (decomposition above)
5. Boiling point (ºC, normal pressure ): Undetermined
6. Boiling point (ºC, 101.3kpa): 260
7. Refractive index: Undetermined
8. Flash point (ºC) : Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): 790
11. Vapor pressure (mmHg, 20ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/water) distribution Log value of coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): 12.6 (g /m3)
19. Solubility: Slightly soluble in water, insoluble in gasoline, soluble in ethanol, chloroform, acetone, methanol, and xylene.
Toxicological data
- Acute toxicity: Rat oral LD50: 323mg/kg
- Mouse oral LD5O: 150mg/kg
- Rabbit oral LD5O: 250mg/kg
- This product is toxic and irritating in contact with skin.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 65.38
2. Molar volume (cm3/mol): 191.0
3. Isotonic specific volume (90.2K): 493.3
4. Surface tension (dyne/cm): 44.4
5. Dielectric constant:
p>
6. Dipole moment (10-24cm3):
7. Polarizability: 25.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.4
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecule polar surface area 50.4
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 225
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents. Slightly soluble in water, soluble in ethanol, chloroform, hot benzene, hot toluene, and easily soluble in dilute inorganic acids. Its aqueous solution is strongly alkaline. Stable in air.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. The production process of diphenylguanidine in my country is the condensation of carbon disulfide and aniline to produce diphenylthiourea, which is then made from diphenylthiourea, lead oxide, ammonium hydroxide and ammonium sulfate at 40-70°C. The sulfate of diphenylguanidine is extracted, filtered and neutralized to obtain a semi-finished product. After drying and screening, the finished product accelerator D is obtained. The differences between the production processes are mainly concentrated in the step of synthesizing diphenylguanidine from diphenylthiourea. In the early days, the lead oxide method was used in China. Due to poor product quality, dust pollution (lead dust) from operators, and three waste treatments of the mother liquor, the yield was low. and other problems, this method was basically discontinued in the late 1990s. This method uses lead oxide as the desulfurization agent, ammonia water as the amination agent, and ammonium sulfate as the extraction agent for diphenylguanidine. There are problems in terms of raw material consumption and environmental protection.


Switching to zinc oxide can improve the original production process. In the mid-1990s, there were reports on the zinc oxide method and the iron oxide method in China, but they were not popular due to various reasons and have been eliminated. All domestic diphenylguanidine manufacturers now use the oxygen method to produce diphenylguanidine, that is, diphenylthiourea reacts with oxygen and ammonia for 8 hours to obtain diphenylguanidine. Raw material consumption quota: diphenylthiourea 1250kg/t, oxygen 240m3, copper acetate (catalyst) 0.86kg/t, ammonia 1000kg/t, water 252kg/t.
2.First, add the required amount of ethanol and water to the pressure-resistant reactor, and pass ammonia at room temperature for 1 hour to make the ammonia in the solution saturated. , then add diphenylthiourea while stirring, and then pass ammonia for 15 minutes. Adjust the zinc oxide into a thin paste and slowly add it dropwise into the reaction kettle for about 1 hour. Finally, seal the reaction kettle, pass ammonia to increase the pressure to the required value, react at 38-40°C for 3 hours, and continue to raise the temperature to React at a certain temperature and for a certain time. After the reaction is completed, release the pressure, discharge materials, filter, and wash with alcohol. The filtrate and washing liquid are combined and distilled to recover part of the ethanol. Since diphenylguanidine is only slightly soluble in water, as the distillation proceeds, an increasing amount of crude diphenylguanidine precipitates from the bottom of the kettle, and stops after the ethanol is basically evaporated. Then add a small amount of water to it, add 1:1 hydrochloric acid dropwise with constant stirring, so that diphenylguanidine generates soluble diphenylguanidine hydrochloride. When the pH value of the solution reaches 2 to 3, stop adding hydrochloric acid dropwise, and remove it. Filter to remove by-products and impurities, and the filtrate is neutralized with 10% NaOH. At this time, refined white diphenylguanidine precipitates. Then filter, wash and dry the product.
3.N,N’-diphenylthiourea, ammonia and oxygen can also be used as raw materials, and divalent copper This product is produced by the oxygen method using ions as catalysts. The synthesis reaction formula is as follows下注br />
First, copper salt and ammonium hydroxide, ammonia and N,N ‘-Diphenylthiourea reacts to form the complex diammine N,N’-diphenylthiourea copper (II) hydroxide and ammonium salt. Then N,N’-diphenylthiourea undergoes oxygen oxidative desulfurization and ammonia reaction to obtain the products diphenylguanidine and ammonium sulfite. Ammonium sulfite continues to be oxidized by oxygen to form ammonium sulfate.
Purpose
Mainly used as a medium-speed accelerator for natural rubber and synthetic rubber. It is commonly used as an active agent for thiazole, thiuram and hypoiodoyl accelerators. When used together with accelerators DM and TMTD, it can be used for continuous vulcanization. When the second accelerator of thiazole accelerator is used, the aging resistance of the vulcanized rubber is reduced, and an appropriate antioxidant must be used. In neoprene, this product acts as a plasticizer and peptizer. This product is not suitable for white or light-colored products and rubber products in contact with food. Mainly used in the manufacture of tires, rubber sheets, shoe soles, industrial products, hard rubber and thick-walled products. As the second accelerator, the dosage is 1-2 parts. When used as the second accelerator of thiazole accelerator, the general dosage is 0.1-0.5 parts. Chromium diphenylguanidine, a derivative of diphenylguanidine, is an efficient rust inhibitor. Diphenylguanidine is also used as a plastic cross-linking agent, temperature indicator material, ore flotation additive, coating additive, polishing material additive, metal analysis reagent and building materials additive.
It is used as a medium-speed and high-temperature vulcanization accelerator for natural rubber, synthetic rubber and rubber-type adhesives. It is also used as a thiuram, Active agent for thiazole and sulfenamide accelerators. The critical vulcanization temperature is 141°C. Vulcanized rubber has good aging resistance, but poor vulcanization flatness. Mainly used for manufacturing tires, rubber sheets, shoe soles, hard rubber products and thick-walled products. It can thicken latex and cause gelation, so it is not suitable for latex or white or light-colored products.
Products. As the second accelerator, the dosage is 1-2 parts. When used as the second accelerator of thiazole accelerator, the general dosage is 0.1-0.5 parts. Chromium diphenylguanidine, a derivative of diphenylguanidine, is an efficient rust inhibitor. Diphenylguanidine is also used as a plastic cross-linking agent, temperature indicator material, ore flotation additive, coating additive, polishing material additive, metal analysis reagent and building materials additive.
It is used as a medium-speed and high-temperature vulcanization accelerator for natural rubber, synthetic rubber and rubber-type adhesives. It is also used as a thiuram, Active agent for thiazole and sulfenamide accelerators. The critical vulcanization temperature is 141°C. Vulcanized rubber has good aging resistance, but poor vulcanization flatness. Mainly used for manufacturing tires, rubber sheets, shoe soles, hard rubber products and thick-walled products. It can thicken latex and cause gelation, so it is not suitable for latex or white or light-colored products.