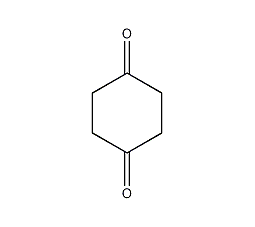
Structural formula
| Business number |
0715 |
| Molecular formula |
C6H8O2 |
| Molecular weight |
112.13 |
| label |
Tetrahydroquinone,
Cyclohexane-1,4-dione,
Tetrahydroquinone,
1,4-Dioxocyclohexane,
pharmaceutical intermediates,
Ester cyclic compounds and their derivatives
|
Numbering system
CAS number:637-88-7
MDL number:MFCD00001606
EINECS number:211-306-0
RTECS number:None
BRN number:774152
PubChem number:24857482
Physical property data
1. Properties: Colorless crystals.
2. Density (g/mL, 25/4℃): 1.0861
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 77-78
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): 132 (2666pa)
7. Refractive index: Undetermined
8. Flash point (ºC): 132
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: soluble in water, ethanol, ether, Acetone, benzene and chloroform.
Toxicological data
None yet
Ecological data
Do not allow undiluted or large quantities of products that are slightly hazardous to water to come into contact with groundwater, waterways or sewage systems. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 27.93
2. Molar volume (cm3/mol): 99.4
3. Isotonic specific volume (90.2K ): 251.0
4. Surface tension (dyne/cm): 40.5
5. Polarizability (10-24cm3):11.07
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -0.6
2. HydrogenNumber of bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Tautomerism Number of bodies: 4
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 98.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. No Determine the number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stay away from oxides.
2. Found in flue-cured tobacco leaves.
Storage method
Store in an airtight container, refrigerated. Store away from oxidizing agents.
Synthesis method
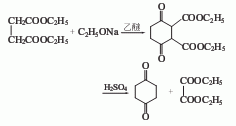
1. Add the prepared sodium ethoxide to the reaction kettle, then add diethyl ether and diethyl succinate, reflux in water for 3 days, and recover the diethyl ether; then cool to room temperature, add 10% dilute sulfuric acid to adjust the pH =2, filter out the crystals, wash with water, and dry to obtain crude diethyl succinate; recrystallize the crude product with ethanol (melting point 127-129°C) to obtain pure product. Then put diethyl succinate back into the flask, add a mixture of concentrated sulfuric acid, water, and ethanol, reflux in oil for 5 days, cool, and neutralize with ammonia to pH = 8; then extract with chloroform 4 times , recover the chloroform to obtain the crude product; then distill the crude product under reduced pressure, pour the distillate into cold petroleum ether, filter and dry to obtain the 1,4-cyclohexanedione product.
2. Tobacco: FC, 18 .
3. Preparation method:
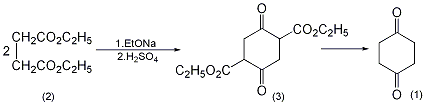
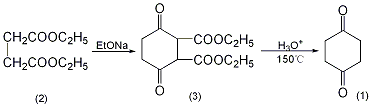
Diethyl 2,5-dioxo-1,4-cyclohexanate (3): In a reaction bottle equipped with a stirrer and a reflux condenser, add 900 mL of absolute ethanol and add in batches 92g (4 mol) of metallic sodium. After adding, heat and reflux to complete the reaction of metallic sodium. After cooling slightly, add 348.4g (2mol) of diethyl succinate at one time (pay attention to prevent overflow), stir and reflux for 24 hours. After recovering ethanol under reduced pressure, add 2000 mL of 2 mol/L sulfuric acid and stir vigorously for 3 to 4 hours. Filter, wash the filter cake with water three times, and dry to obtain 180-190g of crude product, mp 126-128°C. Recrystallize with 1500 mL of ethyl acetate to obtain 160 to 168 g of 2,5-dioxo-1,4-cyclohexanedioic acid diethyl ester (3), mp 126.5 to 128.5°C. The mother liquor is concentrated, and 5.7g can be recovered, with a total yield of 64% to 68%. 1,4-Cyclohexanedione (1): Add 170g (0.66mol) of the above compound (3) and 170mL of water into the pressure reactor, and raise the temperature to 185~195℃ (about 90min). After the heat preservation reaction for 10 to 15 minutes, immediately remove the heat source, quickly cool to room temperature, open the reaction kettle, and pour out the reaction solution to obtain a yellow-orange liquid. Add an equal amount of ethanol, evaporate the solvent under reduced pressure, and then distill under reduced pressure to collect the 130-133°C/2.66kPad fraction (immediately solidified) to obtain 60-66g of 1,4-cyclohexanedione (1), yield 81%~89%. Recrystallize with carbon tetrachloride to obtain pure product. [1]
4. Preparation method:

1,4-Cyclohexanedione-2, 3-Dicarboxylic acid ethyl ester (3): In a reaction bottle equipped with a stirrer and a reflux condenser, add 1000 mL of absolute ethanol, and add 92 g (4 mol) of clean metallic sodium in batches. The reaction is exothermic and can be used if necessary. Water bath cooling. After the metallic sodium has completely reacted, add 350g (2mol) of diethyl succinate (2) in batches and reflux for 25 hours under stirring. A large amount of solid precipitated during the reaction. The ethanol is distilled off. Cool and neutralize to weak acidity with 10% dilute sulfuric acid. Crush the lump, suction filter, wash thoroughly with cold water, and dry to obtain 1,4-cyclohexanedione-2,3-dicarboxylic acid ethyl ester ① (3) 130g, mp126~128 ℃. The yield is 50%. 1,4-Cyclohexanedione (1): In a reaction bottle equipped with a stirrer and a reflux condenser, add 130g (0.508mol) of compound (3), 4L of water, 750g of phosphoric acid, and 100mL of ethanol, and conduct a reflux reaction for 90 hours. Concentrate to half the volume under reduced pressure and extract repeatedly with chloroform. Chloroform was evaporated under reduced pressure to obtain crude product. Distill under reduced pressure, collect the fraction at 125~135℃/2.67kPa, and solidify after cooling to obtain 1,4-cyclohexanedione ② (1) 29g, mp74~76℃, yield 52% . Note: ① The possible mechanism of this reaction is as follows. ② React 1,4-cyclohexanedione-2,3-dicarboxylic acid ethyl ester (3) with an equal amount of water at 4.5~5.9MPa and 180~185℃. The yield can reach more than 65%. [2]
Purpose
This product is used in organic synthesis, synthesis of medicine, electrical conductor materials, etc. It is also a universal reagent.
Resource:allhdi.com