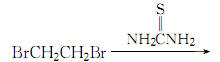
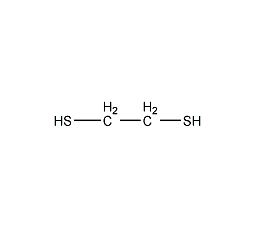
Structural formula
| Business number |
05KB |
| Molecular formula |
C2H6S2 |
| Molecular weight |
94.20 |
| label |
dithioethylene glycol,
Ethylene glycol,
1,2-Ethanedithiol,
1,2-Dimercaptoethane,
Dithioglycol,
Ethylene mercaptan,
Organic Synthesis
|
Numbering system
CAS number:540-63-6
MDL number:MFCD00004892
EINECS number:208-752-3
RTECS number:KI3325000
BRN number:505946
PubChem ID:None
Physical property data
1. Properties: transparent to light green liquid
2. Density (g/ cm3, 25/4℃): 1.123
3 . Relative vapor density (g/cm3, air=1): Undetermined
4. Melting point (ºC): -41
5. Boiling point (ºC, normal pressure): 144-146
6. Boiling point (ºC, 8kPa): Undetermined
7. Refractive index: 1.558
8. Flash point (ºF): 122
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 55.1ºC): Undetermined
13. Heat of combustion (KJ/ mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water ( Log value of the partition coefficient (octanol/water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/ V): Undetermined
19. Solubility: Insoluble in water, miscible in grease,soluble in ethanol, ether, acetone, benzene and alkali solution.
Toxicological data
Acute toxicity: Mouse oral LD50: 342mg/kg, no detailed description except the lethal dose;
Mouse intraperitoneal LC50: 50mg/kg, no detailed description except the lethal dose;
Mouse intravenous LC50: 56200ug/kg, no details except lethal dose;
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 27.10
2. Molar volume (cm3/mol): 89.6
3. Isotonic specific volume (90.2K ): 215.4
4.Surface tension (dyne/cm): 33.3
5. Polarizability (10-24cm3): 10.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 2
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Use and store according to specifications. It will not decompose and avoid contact with oxides.
2. The reagent has a smell and inhalation can cause chest pain, headache, nausea, and pulmonary edema. LD50 342 mg/kg; should be operated in a fume hood.
Storage method
Store refrigerated at 0-5°C
Synthesis method
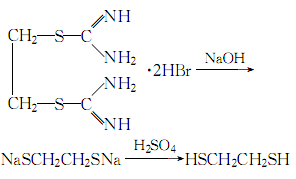
1. First, diisothioureaethane bromide is synthesized from dibromoethane and thiourea, and then treated with sodium hydroxide and sulfuric acid to obtain 1,2-dimercaptoethane.
2.Heat thiourea and 95% ethanol and reflux until clear, stop heating, and slowly add dibromoethane (with constant stirring) The dosage of dibromoethane is 1/2 of the mass of thiourea). The reaction ends after 2 hours. Add the precipitated crystals to 25% to 30% sodium hydroxide solution under stirring, reflux for 6 hours, replace with a distillation device, and slowly add dilute sulfuric acid solution until the reactant becomes acidic to the Congo red test paper. , add 20% more acid. After adding, carry out steam distillation, let stand and separate the layers, separate the oil layer, extract the water layer with ether, combine the recovered ether layer with the oil layer, and dry with anhydrous calcium chloride
After drying, distill under reduced pressure, collect the fractions at 60-145°C at 2.67MPa, and then perform distillation under normal pressure to collect the fractions at 148-150°C, which is the finished product.
The process reaction formula is:
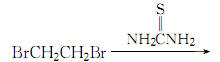
Purpose
1. Used in organic synthesis and biochemical research. Used as metal complexing agent.
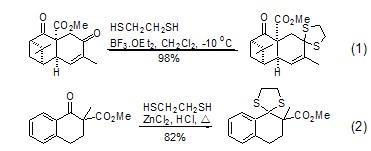
2. The condensation reaction of 1,2-ethanedithiol with aldehydes, ketones and acetals produces 1,3-dithiapentane, which is used for carbonyl protection (Formula 1, Formula 2)[1,2]. Its stability, condensation selectivity and carbonyl regeneration conditions are similar to those of 1,3-propanedithiol[3]. Esters and lactones can be protected with ketene dithioacetals and/or dithioprolactones.
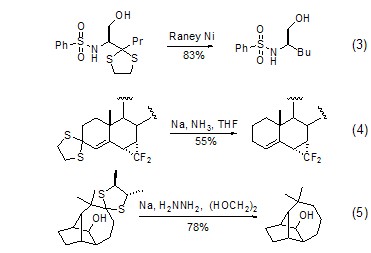
1,3-Dithiapentane Under the conditions of Raney nickel, sodium amide or sodium/hydrazine, the direct desulfurization reaction reduces C=O to CH2 (Formula 3~Formula 5)[4] .
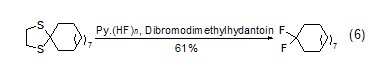
In pyridine hydrofluoric acid and mild Under the action of oxidants, 1,2-ethanedithiol generates geminal difluoride compounds (formula 6)[5]. 1,3-Dithiapentane reacts slowly and has a lower yield.
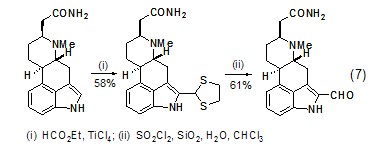
Acid chlorides, acid anhydrides, esters and The orthoester is treated with 1,2-ethanedithiol and Lewis acid to generate the electrophilic 1,3-Dithiolenium cation, which in turn can react with different types of carbon nucleophiles. In the presence of primary carbamoyl, indole-selective formylation reaction can occur (Formula 7)[6].
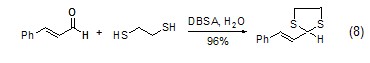
In water, use dodecane As a catalytic system, DBSA can react disulfide acetals and can be used to protect carbonyl compounds (Formula 8) in aqueous media [7].
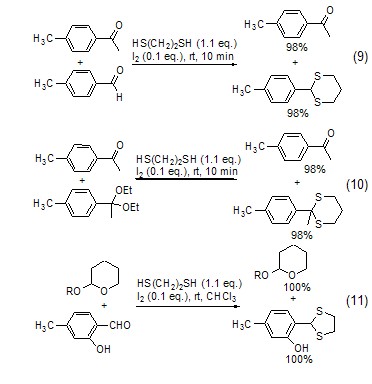
Under the catalysis of iodine, 1 , 2-ethanedithiol can selectively react with ketones and aldehydes, ketal alcohols and ketones, aldehydes and THP ethers, esters and aldehydes (Formula 9~Formula 11)[8].



![1,7-dioxspiro[5,5]undecane structural formula](https://www.bdmaee.net/wp-content/uploads/2024/03/cc894e8b5a3f4e168f40c3dd5fe108aa.gif)
![1,4-dioxspiro[4,5]decane structural formula](https://www.bdmaee.net/wp-content/uploads/2024/03/44cc068911a24a5f9cfb1c19af12dbc1.gif)