2-Bromobutane 2-Bromobutane
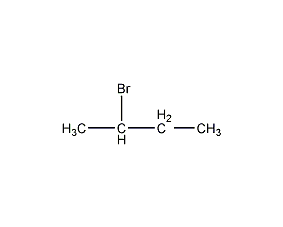

Structural formula
| Business number | 01NG |
|---|---|
| Molecular formula | C4H9Br |
| Molecular weight | 137.02 |
| label |
2-Bromobutane, Bromobutane, sec-butyl bromide, sec-butane bromide, Methylethylmethane bromide, 1-Bromo-1-methylpropane, 2-Butyl bromide, sec-Butyl Bromide, CH3CH2CHBrCH3, Halogenated hydrocarbon solvents, aliphatic compounds |
Numbering system
CAS number:78-76-2
MDL number:MFCD00000156
EINECS number:201-140-7
RTECS number:EJ6228000
BRN number:505949
PubChem number:24851806
Physical property data
1. Properties: colorless liquid with pleasant smell.
2. Density (g/mL, 20/4℃): 1.2585
3. Relative density (25℃, 4℃): 1.2510
4 . Melting point (ºC): -111.9
5. Boiling point (ºC, normal pressure): 91.2
6. Refractive index at room temperature (n25): 1.4342
7. Refractive index (n20ºC): 1.4367
8. Flash point (ºC): 21 (open cup)
9. Solubility parameter ( J·cm-3)0.5: 17.514
10. van der Waals area (cm2·mol-1): 8.250×109
11. van der Waals volume (cm3·mol-1 ): 58.900
12. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -120.3
13. Gas phase Standard entropy (J·mol-1·K-1): 362.15
14. Gas phase standard hot melt (J·mol– 1·K-1): 112.76
15. Liquid phase standard claims heat (enthalpy) (kJ·mol-1) : -154.8
16. Liquid phase standard hot melt (J·mol-1·K-1): 169.4
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Can be compared with Miscible with ethanol, ether, acetone and benzene, but insoluble in water.
Toxicological data
1. Chronic toxicity/carcinogenicity:
Mouse abdominal cavity TDLO: 3000 mg/kg/8W-I2, irritating to eyes and skin.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 28.27
2. Molar volume (cm3/mol): 108.1
3. Isotonic specific volume (90.2K ): 240.8
4. Surface tension (dyne/cm): 24.6
5. Polarizability (10-24cm3):11.20
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 19.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Flammable liquid, highly flammable, easily burns and decomposes when exposed to open flames or high heat, releasing toxic gases. Avoid breathing vapors.
Storage method
This product should be sealed and stored in a cool, dark place.
Synthesis method
It is obtained by reacting sec-butanol with hydrobromic acid in the presence of sulfuric acid. Slowly add concentrated sulfur to hydrobromic acid to mix, then add sec-butanol, reflux for 2-3 hours, distill out bromide, separate the distillate into layers, take the organic phase and dilute it with water, concentrated hydrochloric acid, water, and sodium carbonate in sequence. solution, washed with water, and dried with anhydrous calcium chloride. Finally, distillation collects the 90.5-92.5°C fraction to obtain the finished product.
Purpose
Solvent, organic synthesis intermediate.