2-Butyne-1,4-diol 2-Butyne-1,4-diol
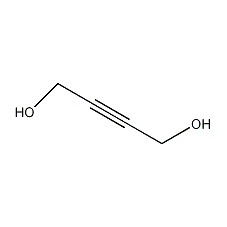
Structural formula
| Business number | 031Q |
|---|---|
| Molecular formula | C4H6O2 |
| Molecular weight | 86.09 |
| label |
Butynediol, 1,4-Butynediol, 1,4-dihydroxy-2-butyne, dihydroxydimethylacetylene, bishydroxymethylacetylene, 1,4-Dimethoxyacetylene, Butynediol, Multifunctional solvents, preservative, brightener |
Numbering system
CAS number:110-65-6
MDL number:MFCD00002915
EINECS number:203-788-6
RTECS number:ES0525000
BRN number:1071237
PubChem number:24891548
Physical property data
1. Properties: Colorless to slightly yellow flaky crystals, with a mellow aroma and easy deliquescing. [1]
2. Melting point (℃): 58[2]
3. Boiling point (℃): 238 [3]
4. Relative density (water=1): 1.07~1.2[4]
5. Saturation Vapor pressure (kPa): 0.133 (102℃)[5]
6. Octanol/water partition coefficient: -0.93[6]
7. Flash point (℃): 128 (OC) [7]
8. Ignition temperature (℃): 410[8 ]
9. Explosion upper limit (%): 35.7[9]
10. Explosion lower limit (%): 2.3 [10]
11. Solubility: Easily soluble in water, easily soluble in methanol and ethanol, insoluble in ether, benzene and chloroform. [11]
12. Boiling point (ºC, 13.33kPa): 194
13. Boiling point (ºC, 1.33kPa): 141
14. Boiling point (ºC, 0.13kPa): 101
15. Flash point (ºC): 248
16. Solubility (g/100mL, 0ºC, water): 121
17. Solubility (g/100mL, 25ºC, water): 374
18. Solubility (g/100mL, 25ºC, ethanol): 83
19. Solubility (g/100mL, 25ºC, in acetone): 70
20. Solubility (g/100mL, 25ºC, in ether): 2.6
21. Solubility (g/ 100mL, 25ºC, benzene): 0.04
22. Refractive index at room temperature (n20): 1.4804
Toxicological data
1. Acute toxicity: guinea pigs LD50: 130mg/kg; rats LD50: 105mg/kg
mice LD50: 105mg/kg; rabbit mouth LD50: 150mg// KG
Mouse inhaled LCLO: 150mg/m3/2h; rats inhaled LCLO: 150mg/m3/2H
2. Acute toxicity [12] </ LD50: 125mg/kg (rat oral)
3. Irritation No information available
Ecological data
1. Ecotoxicity[13] LC50: 49.3~58.3mg/L (96h) (fathead minnow)
2. Biodegradability No data available
3. Non-biodegradability No data available
Molecular structure data
1. Molar refractive index: 21.65
2. Molar volume (cm3/mol): 72.8
3. Isotonic specific volume (90.2K ): 202.9
4. Surface tension (dyne/cm): 60.0
5. Polarizability (10-24cm3): 8.58
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -1.1
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 66
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: React with acid or acid anhydride to form monoester, which can undergo electrolytic oxidation, isomerization, halogenation and other reactions.
2. It has anesthetic and irritating effects on the upper respiratory tract, eye mucosa and skin. Long-term inhalation of vapor with a concentration of 0.008~0.01mg/L will cause lesions in the central nervous system, liver function, internal tissues, etc. The oral LD of rabbits and guinea pigs is 0.125mL/kg. The maximum allowable concentration in the air in the production workshop is <0.001mg/L. The equipment is required to be sealed, the workshop should be forcedly ventilated, and operators should wear protective equipment.
3. Stability[14] Stable
4. Incompatible substances[15] Strong oxidants, strong bases, acid anhydrides, acid chlorides, mercury salts, strong acids, alkaline earth metals, hydroxides and halides, etc.
5. Conditions to avoid contact [16] Friction and impact
6. Polymerization hazard[17] No polymerization
Storage method
1. Reagent-grade products are packaged in brown glass bottles, 500g per bottle. It can also be packed in plastic bottles, 1kg per bottle. Industrial products are packaged in 1kg plastic bottles, 20kg plastic drums or 100kg iron drums. Should be stored in a cool, ventilated and dry place. Protect from heat, sun and moisture. Store and transport according to regulations on toxic substances.
2. Storage precautions [18] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Using the acetylene-formaldehyde synthesis method, acetylene containing 80%-90% is compressed to a pressure of 0.4-0.5MPa, preheated to 70-80°C, and sent to the reactor, using butynyl ketone as the catalyst, and formaldehyde React at 110-112°C to obtain crude product. The reaction product is concentrated and refined to obtain the finished product, and propargyl alcohol is produced as a by-product.
![]()
2. Paraformaldehyde normal pressure Solvent method: Use paraformaldehyde as raw material and cyclohexanone as solvent. In the presence of catalyst copper acetylene, acetylene is fed into the reactor and the temperature is maintained at 115-120°C. Stop flowing acetylene after formaldehyde is completely converted. The catalyst is filtered off, and the reaction solution is concentrated and recrystallized to obtain crystalline butynediol.
Purpose
1. Butynediol can produce a series of important organic products such as butenediol, butylene glycol, n-butanol, dihydrofuran, tetrahydrofuran, γ-butyrolactone, pyrrolidone, etc., and can further produce synthetic plastics , synthetic fibers (nylon-4), artificial leather, medicines, pesticides, solvents (N-methylpyrrolidone) and preservatives. Butynediol itself is a good solvent and is used as a brightener in the electroplating industry.
2. Used as secondary brightener for nickel plating. Combined with primary brightener, a fully bright, flat and ductile coating can be obtained.
3. Used in organic synthesis and as electroplating brightener. [19]