2-Chloro-4-nitrotoluene 2-Chloro-4-nitrotoluene
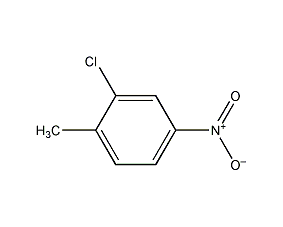

Structural formula
| Business number | 03E4 |
|---|---|
| Molecular formula | C7H6ClNO2 |
| Molecular weight | 171 |
| label |
None yet |
Numbering system
CAS number:121-86-8
MDL number:MFCD00007210
EINECS number:204-501-7
RTECS number:XS9100000
BRN number:1817924
PubChem number:24869631
Physical property data
1. Character: needle-like crystal.
2. Refractive index (nD20):1.5470
3. Melting point (℃): 68℃
4. Boiling point (ºC,2.0kpa):260℃
5. Solubility:Soluble in ether, ethanol and acetic acid, slightly soluble in hot water. Can evaporate with water vapor.
Toxicological data
None
Ecological data
None
Molecular structure data
5. Molecular property data: 1. Molar refractive index:42.51 2. Molar Volume(m3/mol):129.5 3. Isotonic specific volume(90.2K):336.2 4. Surface tension(dyne/cm):45.4 5. Dielectric constant: 6. Dipole moment(10-24cm3): 7. Polarizability:16.85
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 157
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Storage method
None
Synthesis method
1. Introduction to production methods
Obtained from chlorination of p-nitrotoluene. First add dry pure p-nitrotoluene to the acid-resistant ceramic three-necked bottle and heat it in an external water bath to 55℃, then Add a small amount of iodine and iron filings, 50-60℃Pour in chlorine gas. After a few hours, the chlorine gas will absorb up to The theoretical quantity stops. At this time, there are reactants precipitating in the bottle. Heat the reactants with steam to melt them and then extract them. New Roman”>5%Wash twice with dilute hydrochloric acid. Then use 1%Wash once with sodium carbonate solution, and finally Wash once with hot water, stir the oil into cold water to form granular material, filter, wash with cold water, rinse in 40℃Blow dry to get the finished product. In industrial production, the operation process can be carried out in a dry reaction pot. Put the molten nitrotoluene into the reaction pot and add iron wire and iodine tablets (or add ferric chloride). style=”FONT-SIZE: 10pt; COLOR: #333333; FONT-FAMILY: Times New Roman”>58-62℃Pour in an equal molar amount of chlorine, wash with hot water and neutralize with sodium carbonate, pour into ice water, stir to precipitate crystals, filter and dry in the shade The finished product is obtained.
Purpose
2. Purpose
Organic synthesis intermediates. Used in the production of green wheat flour.
y: 宋体”>℃Pour in an equal molar amount of chlorine, wash with hot water and neutralize with sodium carbonate, then pour into ice water, stir to precipitate crystals, filter, and dry in the shade to obtain the finished product.
Purpose
2. Purpose
Organic synthesis intermediates. Used in the production of green wheat flour.