2-Chlorotoluene 2-Chlorotoluene

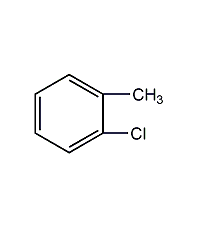
Structural formula
| Business number | 0296 |
|---|---|
| Molecular formula | C7H7Cl |
| Molecular weight | 126.58 |
| label |
o-chlorotoluene, 2-Chloro-1-methylbenzene, 2-Chlorotoluene, 1-Chloro-2-toluene, o-Chlorotoluene, 2-Chloro-1-methylbenzene, rubber solvent, Solvent for synthetic resin, Aromatic halogen derivatives |
Numbering system
CAS number:95-49-8
MDL number:MFCD00000562
EINECS number:202-424-3
RTECS number:XS9000000
BRN number:1904175
PubChem number:24856063
Physical property data
1. Properties: Colorless and transparent liquid with an almond-like odor.
2. Boiling point (ºC, 101.3kPa): 159.2
3. Melting point (ºC, purity 99.6% (mol)): -35.59
4. Relative density (g/mL, 20/4ºC): 1.0817
5. Relative density of steam (g/mL, air=1): 4.37
6. Refractive index (n20ºC) : 1.5267
7. Viscosity (mPa·s, 15ºC): 1.037
8. Flash point (ºC, opening): 57.8
9. Heat of evaporation (KJ/kg, 158.07ºC): 304.0
10. Body expansion coefficient (K-1, 30ºC): 0.00092
11. Solubility: It can be dissolved in benzene, toluene, alcohol, ether, ketone, butyl acetate, 1,2-dichloroethane, chloroform and other organic solvents. The solubility in water at 25℃ is 0.037%; the solubility of water in o-chlorotoluene is 0.014%.
12. Relative density (20℃, 4℃): 1.0726
13. Refractive index at room temperature (n25): 1.520330
14. Relative density (25℃, 4℃): 1.0872830
15. Liquid phase standard hot melt (J·mol-1·K-1): 198.5
Toxicological data
1. Acute toxicity: Oral LD50 in rats: 3900mg/kg
Oral LD5O in mice: 2500mg/kg
Inhalation LCLO in rats: 4400ppm
LCLO inhaled by mice: 4400ppm
LCLO inhaled by guinea pigs: 4400ppm
2. The physiological effects are similar to those of chlorobenzene. They are mainly absorbed through the respiratory tract and are harmful to mucous membranes (especially the conjunctiva). Has a stimulating effect. Damage to the central nervous system and internal organs. Skin contact can cause erythema and bullae and even eczema. The maximum allowable concentration in the workplace is 250mg/m3 (US).
Ecological data
This substance is harmful to the environment and it is recommended not to let it enter the environment. Special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 35.97
2. Molar volume (cm3/mol): 117.6
3. Isotonic specific volume (90.2K ): 280.7
4. Surface tension (dyne/cm): 32.4
5. Dielectric constant:
6. Dipole moment (10-24cm3 ):
7. Polarizability: 14.26
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 70.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides. Dry o-chlorotoluene is non-corrosive to metals, but copper containers are not suitable. Under the influence of heat and moisture, highly corrosive hydrogen chloride is gradually released. Chemical properties: When heated to 260°C with air in sodium hydroxide solution under pressure, o-chlorobenzoic acid, o-chlorobenzaldehyde and a small amount of formic acid, acetic acid and oxalic acid are generated. In the presence of copper, it is heated to 350~360°C with sodium hydroxide solution under pressure to generate o-cresol and m-cresol. It reacts with ammonia under pressure and in the presence of a catalyst to produce o-toluidine. Use ferric chloride, antimony chloride, and aluminum amalgam as catalysts to perform chlorination reaction to generate dichlorotoluene and trichlorotoluene. Chlorination is carried out under the catalysis of light or phosphorus pentachloride, and a chlorine substitution reaction occurs on the side chain to generate o-chlorobenzyl chloride, o-chlorodichlorobenzyl and trichlorobenzyl. When heated with aluminum trichloride and hydrogen chloride, part of the isomerization occurs and is converted into m-chlorotoluene and p-chlorotoluene. Sulfonation with fuming sulfuric acid produces 4-chloro-3-methylbenzenesulfonic acid. 2.This product is toxic; it can evaporate together with water vapor. The operation site should be well ventilated, the equipment should be sealed, and the operators should wear protective equipment. See parachlorotoluene.
Storage method
Stored sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep sealed. Keep away from fire sources, anti-static and anti-explosion. Store away from oxidizing agents.
Stored in a cool, ventilated warehouse, away from heat sources and fires, and stored separately from oxidants and food additives. When handling, pack and unload lightly to prevent packaging damage. Iron drum packaging. 200kg per barrel. Store and transport according to regulations on toxic chemicals.
Synthesis method
Originated from o-toluidine through diazotization and substitution. Add o-toluidine, hydrochloric acid and water to the reaction pot, stir and heat to 50°C, keep for 0.5h, cool to 0-5°C, add sodium nitrite solution dropwise until the potassium iodide starch test paper turns blue to obtain a diazonium salt solution. In addition, stir water, copper sulfate and sodium chloride evenly, heat to 80°C to dissolve, cool to 40°C, add sodium sulfite solution dropwise, stir for 0.5h, cool and let stand. Separate the upper wastewater. Dissolve the precipitated cuprous chloride with hydrochloric acid, slowly add the above diazonium salt solution, the temperature does not exceed 25°C, stir for 0.5h, let it stand for stratification, discard the water layer, and wash the crude o-chlorotoluene with acid and alkali. Distill under normal pressure and collect the 157-160°C fraction to obtain the finished product. In addition, toluene can also be obtained by aromatic ring chlorination and separation.

Purpose
Used as intermediates for dyes, medicines, organic synthesis, and solvents for rubber and synthetic resins.