2-Hydroxy-4-methoxybenzophenone

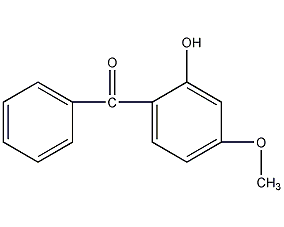
Structural formula
| Business number | 03N3 |
|---|---|
| Molecular formula | C14H12O3 |
| Molecular weight | 228.24 |
| label |
External ray absorber UV-9, 2-hydroxy-4-methoxybenzophenone, Sunscreen No. 2, BP-3, ultraviolet absorber UV-9, HOC6H3(OCH3)COC6H5, UV absorber |
Numbering system
CAS number:131-57-7
MDL number:MFCD00008387
EINECS number:205-031-5
RTECS number:DJ1575000
BRN number:1913145
PubChem number:24879471
Physical property data
1. Properties: light yellow crystal or milky white powder
2. Density (g/mL, 25/4℃): 1.324
3. Melting point (℃): 63 -64
4. Boiling point (ºC, 2.4kpa): 220
5. Solubility: Insoluble in water. Can be dissolved in a variety of organic solvents such as acetone, ethanol, ethyl acetate, methanol
6. Toxicity: low toxicity
7. Stability: good light and thermal stability, Does not decompose at 200℃.
Toxicological data
The oral LD50 of rat is 7.4g/kg body weight. The allowable human intake is 3.3mg/kg.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 64.60
2. Molar volume (cm3/mol): 189.9
3. Isotonic specific volume (90.2K ): 498.5
4. Surface tension (dyne/cm): 47.4
5. Polarizability (10-24cm3): 25.61
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 5
6. Topological molecule polar surface area 46.5
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 258
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Non-flammable, non-corrosive, and has good storage stability.
Storage method
Packed in iron drums, sealed and dryStore dry.
Synthesis method
Using resorcinol methylation method. Using resorcinol as raw material, methylation reaction is carried out with dimethyl sulfate in the presence of sodium hydroxide catalyst, and then the oil vapor is separated, distilled, and then reacted with benzoyl chloride in the presence of aluminum trichloride and chlorobenzene. , finally obtained by hydrolysis, distillation, cooling, decolorization, centrifugation and drying.



Purpose
This product is a UV absorber that can effectively absorb ultraviolet light of 290-400nm, but hardly absorbs visible light, so it is used in light-colored plastic products. It can be used in paints and various plastics, such as cellulose acetate, nitrocellulose, polyvinyl chloride, chlorinated polyvinyl chloride, polystyrene, ABS and polyester, unsaturated polyester, etc. The general dosage of this product is 0.1 to 1.5 parts.
This product is a broad-spectrum ultraviolet absorber with the advantages of high absorption rate, non-toxicity, non-teratogenic effect, and good stability to light and heat. This product can absorb both UV-A and UV-B. It is a Class I sunscreen approved by the U.S. FDA. It is used more frequently in the United States and Europe. It is widely used in sunscreen cosmetics such as sunscreen ointments, creams, honeys, lotions, and oils. It is also It can be used as an anti-discoloration agent for products that change color due to photosensitivity.