2-Methoxy-4-nitroaniline 2-Methoxy-4-nitroaniline
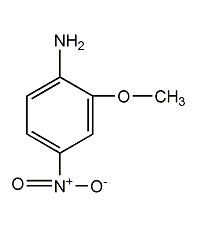

Structural formula
| Business number | 02C9 |
|---|---|
| Molecular formula | C7H8N2O3 |
| Molecular weight | 168.15 |
| label |
Red base B, red bass B, 1-Amino-2-methoxy-4-nitrobenzene, 4-Nitro-o-anisidine |
Numbering system
CAS number:97-52-9
MDL number:MFCD00007363
EINECS number:202-588-6
RTECS number:BZ7170000
BRN number:879619
PubChem number:24846314
Physical property data
- Appearance: yellow or tan powder
- Density (g/mL, 20℃): 1.2112
- Relative vapor density (g/mL , air = 1): not determined
- Melting point (ºC): 137-140
- Boiling point (ºC, normal pressure): not determined
- Boiling point (ºC , KPa): not determined
- Refractive index: not determined
- Flash point (ºC): not determined
- Specific rotation (º): not determined
- Autoignition point or ignition temperature (ºC): Not determined
- Vapor pressure (mmHg, 20.2ºC): Not determined
- Saturation vapor pressure (kPa, ºC ): Undetermined
- Heat of combustion (KJ/mol): Undetermined
- Critical temperature (ºC): Undetermined
- Critical pressure (KPa): Undetermined Determined
- Log value of oil-water (octanol/water) partition coefficient: Undetermined
- Explosion upper limit (%, V/V): Undetermined
- Explosion Lower limit (%, V/V): Undetermined
- Solubility: Insoluble in water, easily soluble in acetone, soluble in ethanol, ethyl acetate, acetic acid and benzene.
Toxicological data
Acute toxicity: rat oral LD50: 997mg/kg
Ecological data
This substance is harmful to the environment. Do not let this substance enter the environment.
Molecular structure data
1. Molar refractive index: 43.71
2. Molar volume (cm3/mol): 127.5
3. Isotonic specific volume (90.2K ): 345.2
4. Surface tension (dyne/cm): 53.6
5. Polarizability (10-24cm3): 17.32
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 81.1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 169
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants, acids, acid anhydrides, and acid chlorides.
Toxic, easily absorbed by the skin and poisoned, but quickly excreted from the urethra. Operators need to wear gloves, masks, and drink plenty of water. OriginalThe material anthranilate is highly toxic, and the maximum allowable concentration in the air is 0.5mg/m3.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from oxidants, acids, acid anhydrides and acid chlorides, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
o-Methoxyaniline is condensed with p-toluenesulfonyl chloride in the presence of sodium carbonate to obtain N-p-toluenesulfonyl o-methoxyaniline, and then nitrated with nitric acid to obtain 4-nitro-2-methoxy-N- For p-toluenesulfonyl aniline, the nitrate is treated with dilute sulfuric acid and hydrolyzed to remove the p-toluenesulfonyl group to obtain the finished product. Add o-methoxyaniline and equimolar p-toluenesulfonyl chloride to water at 85°C, and add sodium carbonate to adjust the pH of the reaction solution to 8. After reaching the end of the reaction, cool to 60°C, adjust the pH to 6 with sulfuric acid, cool to 45°C, and filter to obtain N-p-toluenesulfonyl-o-methoxyaniline with a yield of 99%. Add chlorobenzene to the nitrification pot and put in the above condensation product. Stir and add sodium nitrite at 30-33°C, and then slowly add 82% nitric acid. The adding temperature does not exceed 50°C. Cool to 35°C in about 15 minutes, and then add the condensation product and nitric acid in proportion. The feeding time is 3.5 hours, and the reaction temperature is controlled at 35-45°C. After the addition is completed, react at 50°C for 1.5 hours. Cool to 30°C and filter. The filter cake is washed with chlorobenzene and water, and dried to obtain 4-nitro-2-methoxy-N-p-toluenesulfonylaniline with a yield of 85%. Add this nitrate to 82%-83% sulfuric acid at 78°C. After the addition is completed, keep it at 90°C for 2.5 hours. Dilute with water, add alkali to neutralize to pH 7.5, press filter at 30°C, wash with 5% soda ash solution and water, and dry to obtain 2-methoxy-4-nitroaniline with a yield of 95%. The obtained product is yellow or tan powder. Content (dry basis) ≥98%. Raw material consumption quota: o-methoxyaniline 960kg/t, p-toluenesulfonyl chloride 1540kg/t.
Purpose
Dye intermediates. It is mainly used as the color base for ice dyeing dyes, namely red base B. Used to prepare dyes such as light-fast bright yellow 10GC. It is mainly used for dyeing and printing color development of cotton, linen, silk and polyester fabrics. It is also used to produce fast pigments, maroon IB, golden, black and organic lemon yellow lamp pigments.