2-Methyl-3-butyn-2-ol 2-Methyl-3-butyn-2-ol
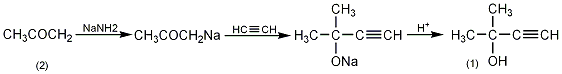
Structural formula
| Business number | 037P |
|---|---|
| Molecular formula | C5H8O |
| Molecular weight | 84.12 |
| label |
3-methylbutynol-3, 2-Methyl-3-butyn-2-ol, 3-Methyl butynol, Dimethyl ethynyl carbinol, Stabilizer for chlorine-containing solvents, Multifunctional solvent |
Numbering system
CAS number:115-19-5
MDL number:MFCD00004467
EINECS number:204-070-5
RTECS number:ES0810000
BRN number:635746
PubChem number:24847925
Physical property data
1. Properties: colorless, aromatic liquid.
2. Density (g/mL, 20/4℃): 0.8618
3. Relative vapor density (g/mL, air=1): 2.49
4. Melting point (ºC): 3
5. Boiling point (ºC, normal pressure): 104
6. Refractive index (20ºC): 1.4207
7. Refractive index (25ºC): 1.4184
8. Flash point (ºC): 25
9. Vapor pressure (kPa, 20ºC): 1.6
10. Vapor pressure (kPa, 52ºC): 10.7
11. Solubility: miscible with water, acetone, benzene, carbon tetrachloride, ethyl acetate, methyl ethyl ketone, fatty acids, petroleum ether, etc. dissolve.
Toxicological data
1. Acute toxicity: Rat oral LD50: 1950mg/kg; mouse oral LD50: 500mg/kg; 1161mg/kg
2. It is harmful if taken orally and may cause severe burns to the eyes.
Ecological data
Mildly hazardous to water.
Molecular structure data
1. Molar refractive index: 24.71
2. Molar volume (cm3/mol): 92.2
3. Isotonic specific volume (90.2K ): 223.3
4. Surface tension (dyne/cm): 34.3
5. Polarizability (10-24cm3): 9.79
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 83.4
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxidizing agents. It is easy to burn and explode when exposed to high heat or open flame. Vapors form explosive mixtures with air and react violently on contact with oxidants.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire, heat sources and anti-static. Protect from direct sunlight. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Equipped with corresponding varieties and quantities of fire-fighting equipment and explosion-proof facilities. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Preparation method:
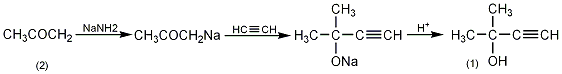
In a reaction bottle equipped with a stirrer, dropping funnel, reflux condenser (ammonia drying tube), and ventilation tube, add 1L of anhydrous ether and 156g (4mol) of very finely ground sodium amide, and cool in an ice-salt bath Slowly add 232g (4mol) of anhydrous acetone (2) dropwise, and complete the addition in about 3 hours. Cool to -10°C and slowly introduce acetylene gas flow for 2 hours to drive out ammonia. Put one end of the air inlet pipe deep into the bottom of the bottle, and connect the other end to the acetylene gas cylinder. The stopper is tied tightly with a metal wire. Cool it in an ice-salt bath and place the entire device on a vibrator. Shake vigorously and react for 10 hours (maintaining an acetylene pressure of 0.7MPa). Down). Open the piston to relieve pressure every 30 minutes to remove ammonia. After the reaction is completed, slowly pour the reactant into 800g of crushed ice, and add 400 mL of 20 mol/L sulfuric acid for acidification while cooling. The organic layer was separated, and the aqueous layer was extracted twice with diethyl ether. The ether layers were combined and dried over anhydrous potassium carbonate. Filter, recover the solvent and then fractionate, collect the fraction at 103-107°C to obtain 135-155g of colorless liquid compound (1), with a yield of 46%. [1]
Purpose
Used as solvents, intermediates, and stabilizers for chlorine-containing solvents.