2-methyl-3-pentanone
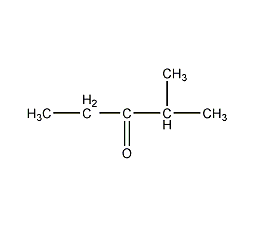

Structural formula
| Business number | 05TL |
|---|---|
| Molecular formula | C6H12O |
| Molecular weight | 100.16 |
| label |
Ethyl isopropyl ketone, Ethyl isopropyl ketone, Isopropyl ethyl ketone, 4-Methyl-3-pentanone, aliphatic compounds |
Numbering system
CAS number:565-69-5
MDL number:MFCD00009314
EINECS number:209-288-4
RTECS number:None
BRN number:1698849
PubChem number:24845695
Physical property data
1. Properties: colorless or light yellow flammable liquid.
2. Boiling point (ºC, 101.3kPa): 112~116
3. Relative density (g/mL, 20/4ºC): 0.8106
4 . Refractive index (n20ºC): 1.3981
5. Flash point (ºC): 11
6. Solubility: slightly soluble in water, easily soluble in ethanol, benzene, miscible Soluble in acetone and ether. Soluble in chloroform.
7. Relative density (25℃, 4℃): 0.8059
8. Refractive index at room temperature (n25): 1.3958
9. Solubility parameter (J·cm-3)0.5: 17.325
10. van der Waals area (cm2 ·mol-1): 9.880×109
11. van der Waals volume (cm3·mol-1): 69.720
12. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3789.95
13. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -286.10
14. Liquid phase standard combustion heat (enthalpy) (kJ ·mol-1): -3750.16
15. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -325.89
16. Liquid phase standard hot melt (J·mol-1·K-1): 207.3
Toxicological data
Irritating to eyes, mucous membranes and skin.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 29.83
2. Molar volume (cm3/mol): 125.0
3. Isotonic specific volume (90.2K ): 273.3
4. Surface tension (dyne/cm): 22.8
5. Polarizability (10-24cm3): 11.82
Compute chemical data
1. Hydrophobic parameter calculation reference� (XlogP): 1.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Rotatable chemical bonds Number: 2
5. Number of tautomers: 3
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 64.6
10. Number of isotope atoms: 0
11. Determine the atoms Number of stereocenters: 0
12. Uncertain number of stereocenters of atoms: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Easily burns when exposed to open flames or high heat.
2. Exist in flue-cured tobacco leaves and mainstream smoke.
Storage method
Seal the secret container and store it in a sealed main container in a cool, dry place.
Synthesis method
1. Tobacco: FC, 40.
Purpose
Used as solvent.