2-Pyridinecarboxylic Acid 2-Pyridinecarboxylic Acid
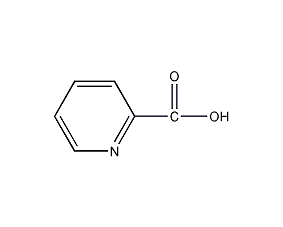

Structural formula
| Business number | 02EZ |
|---|---|
| Molecular formula | C6H5NO2 |
| Molecular weight | 123.11 |
| label |
2-Pyridinecarboxylate, Pyridine-2-carboxylic acid, Picolinic acid, 2-Picolinic acid, Pyridine-2-carboxylic Acid, 2-Picolinic acid, α-picolinic acid |
Numbering system
CAS number:98-98-6
MDL number:MFCD00006293
EINECS number:202-719-7
RTECS number:TJ7344000
BRN number:109595
PubChem ID:None
Physical property data
1. Properties: White or reddish needle-like crystals.
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 134-136
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 30mmHg): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in glacial acetic acid, almost insoluble in ether, Chloroform and carbon disulfide.
Toxicological data
1. Acute toxicity: mouse peritoneal cavity LD50: 360mg/kg; mouse intravenous injection LD50: 487mg/kg; quail oral LD50: 562mg/kg; wild bird oral LD50: 178mg/kg; 2. Other multiple doses Toxicity: Rat peritoneal cavity TDLo: 295mg/kg/8D-I;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 31.27
2. Molar volume (cm3/mol): 95.1
3. Isotonic specific volume (90.2K ): 263.5
4, Surface tension (dyne/cm): 58.7
5, Dielectric constant:
6, Dipole moment (10-24cm 3):
7. Polarizability: 12.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 50.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 114
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with acids, oxidants, reducing agents, and alkalis.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from acidic substances, reducing agents and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Add 50g of 2-methylpyridine to 2500ml of water, heat and stir, add 90g of potassium permanganate, and then add a second portion of 90g of potassium permanganate about 1 hour later. Add 500ml of water and continue heating for 2-2.5 hours until the purple color disappears. Cool, filter out the manganese dioxide precipitate, and wash with hot water. The filtrate was concentrated under reduced pressure and filtered. The concentrated solution was acidified with concentrated hydrochloric acid until Congo red was acidic, evaporated to dryness, and then boiled with 250 ml of 95% ethanol and refluxed for 1 hour. Filter, extract the filter cake with 95% ethanol, combine the ethanol filtrate and extract, and pass in dry hydrogen chloride until crystals begin to precipitate. Cool to 10°C, then add hydrogen chloride to distillate, filter out the picolinate hydrochloride, and obtain 43-44g after drying (the melting point of the hydrochloride is 210-212°C).
Purpose
Organic synthesis intermediates.