2-thiophene formaldehyde Thiophene-2-carboxaldehyde
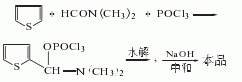

Structural formula
| Business number | 02D4 |
|---|---|
| Molecular formula | C5H4OS |
| Molecular weight | 112.15 |
| label |
Thiophene-2-carbaldehyde, 2-thiophenal, 2-Thenaldehyde, 2-Thiophenaldehyde, 2-Thiophenealdehyde, 2-Thiophenecarbaldehyde, 2-Thiophenecarboxaldehyde, 2-Formyl thiophene, Akosbbs-00003201, thenaldehyde, Heterocyclic compounds |
Numbering system
CAS number:98-03-3
MDL number:MFCD00005429
EINECS number:202-629-8
RTECS number:XM8135000
BRN number:105819
PubChem number:24900117
Physical property data
1. Properties: light yellow liquid.
2. Density (g/mL, 25℃): 1.22
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): 196-198
6. Boiling point (ºC, kPa): Undetermined
p>
7. Refractive index: 1.591
8. Flash point (ºC): 78
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 55ºC): Not determined
12. Saturated vapor pressure (kPa, 25 ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15 . Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V ): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in ethanol, benzene, ether, slightly soluble in water .
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit skin contact, 500mg/24 HREACTION SEVERITY, mild reaction; Standard Dresser test: rabbit eye contact, 100 mgREACTION SEVERITY, moderate reaction; 2. Acute toxicity: rats Oral LD50: 915mg/kg; Mouse oral LD50: 565mg/kg; Guinea pig skin contact LD50: >10mL/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 31.39
2. Molar volume (cm3/mol): 90.5
3. Isotonic specific volume (90.2K ): 235.49
4. Surface tension (dyne/cm): 45.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 12.44
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 17.1
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 72.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Does not decompose under normal temperature and pressure. Avoid contact with oxidants, reducing agents, alkalis, and air.
Toxic, please refer to thiophene for its toxicity and protection methods.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants and alkalis, and avoid mixed storage. Store away from air. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials. 2. Use glass bottles or polyethylene plastic barrels for sealed packaging and protect with nitrogen to prevent oxidation. Store in a cool, ventilated and dry place. Sun protection, waterproof, bump and inversion resistant. Store and transport according to regulations on toxic chemicals.
Synthesis method
It is obtained by condensation of thiophene and dimethylformamide in the presence of phosphorus oxychloride, followed by hydrolysis, neutralization and refinement. Add thiophene and dimethylformamide into the reaction pot, slowly add phosphorus oxychloride below 15°C, gradually raise the temperature to 80-90°C, keep it warm for 3 hours, cool to 30°C, and add ice water for hydrolysis. Then neutralize it with 30% sodium oxyoxide, let it stand and separate into layers. The water layer is extracted with carbon tetrachloride. The extract is washed and dehydrated, the solvent is recovered, and distilled under reduced pressure to obtain 2-thiophene aldehyde.
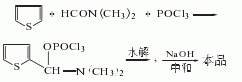
Purpose
It is used as an intermediate for the broad-spectrum anthelmintic drug pyrantel and the drugs cephalosporin 1 and 2.