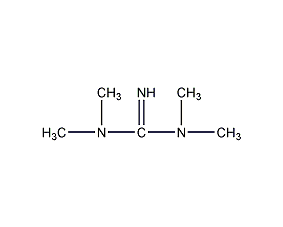
2-Chloro-4,6-bis(ethylamino)-1,3,5-triazine 2-Chloro-4,6-bis(ethylamino)-1,3,5-triazine


Structural formula
| Business number | 03EV |
|---|---|
| Molecular formula | C7H12ClN5 |
| Molecular weight | 201 |
| label |
simazine, Aquazine, Premazine, Symazine, Tafazine, Taphazine, herbicide |
Numbering system
CAS number:122-34-9
MDL number:MFCD00023174
EINECS number:204-535-2
RTECS number:XY5250000
BRN number:10895
PubChem number:24869206
Physical property data
1. Properties: The pure product is white crystal. 2. Density (g/mL, 25/4℃): 1.302
3. Melting point (℃): 226~227
4. Vapor pressure (kpa, 20ºC): 8.13×10-7
5. Solubility: insoluble in water, solubility in methanol is 400ppm, slightly soluble in di Oxyhexanes and ethylene glycol ether.
6. Toxicity: Low toxicity to humans and animals
7. Stability: Stable
Toxicological data
Acute oral LD50>SOOOmg/kg; 80% in rats, acute transdermal LD50 in rabbits >100mg/kg
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 48.05
2. Molar volume (cm3/mol): 151.1
3. Isotonic specific volume (90.2K): 406.4
4. Surface tension (dyne/cm): 52.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 19.05
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: 5
6. Topological molecule polar surface area 62.7
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 131
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
Keep sealed.
Synthesis method
1. Obtained from the reaction of triazine cyanide and ethylamine in the presence of acid acceptor. If water is used as the reaction medium, add the material at about 0°C, then keep stirring at 70°C for 2 hours. If the reaction is carried out in a solvent such as trichlorethylene, the reaction temperature is 30-50°C. Raw material consumption quota: cyanuric chloride (96%) 1020kg/t, monoethylamine (100%) 520kg/t, liquid caustic soda (40%) 100kg/t, trichlorethylene (industrial product) 120kg/t.
2.Use water as the reaction medium, mix cyanuric chloride and ethylamine at about 0℃ in the presence of acid binding agent, and then Prepared by 70°C insulation reaction for 2 hours.
Purpose
1. This product is a highly efficient and selective herbicide, mostly used to control annual and perennial broadleaf weeds and most monocotyledonous weeds propagated by seeds in corn, sorghum, sugarcane, tea gardens, orchards, etc. It can also be used on railways , highways, oil fields and biocidal weeding. Pesticide residue analysis standards. herbicide.
2.S-triazine herbicides. It is easily adsorbed on the surface of the soil, forming a poisonous soil layer. The roots of shallow-rooted weed seedlings absorb the pesticide and are killed. Less effective on perennial or deep-rooted weeds with deeper root systems. Used to control annual or perennial broadleaf weeds and most monocotyledonous weeds propagated by seeds in corn, sugar cane, sorghum, tea trees, rubber, orchards and nurseries; it can significantly inhibit perennial weeds propagated by rhizomes or root buds. Effect; appropriately increase the dose and can also be used as a biocidal herbicide on forest fire roads, along railway roadbeds, courtyards, warehouse storage areas, oil tank areas, lumberyards, etc.