5,5-Dimethyl-1,3-cyclohexanedione 5,5- Dimrthyl-1,3-cyclohexanedione
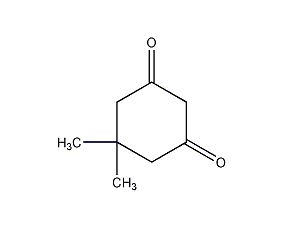
Structural formula
| Business number | 03KA |
|---|---|
| Molecular formula | C8H12O2 |
| Molecular weight | 140.18 |
| label |
1,1-Dimethylcyclohexanedione, 1,1-dimethylcyclohexane-3,5-dione, Damidon, Dimidone, dimethyldihydroresorcinol, dimethylcyclohexanedione, aldehyde reagent, Alicyclic compounds |
Numbering system
CAS number:126-81-8
MDL number:MFCD00001588
EINECS number:204-804-4
RTECS number:GV0345000
BRN number:471489
PubChem number:24864009
Physical property data
1. Properties: white to green-yellow needle-like or columnar crystals
2. Melting point: 148-149°C
3. Solubility: soluble in methanol, ethanol, chloroform , benzene, acetic acid and 50% alcohol-water mixture
4. Stability: dry crystallization is stable, but its solution can decompose and oxidize even under light shielding
Toxicological data
Acute toxicity data
Rat oral LD5: >500 mg/kg
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 37.25
2. Molar volume (cm3/mol): 137.8
3. Isotonic specific volume (90.2K): 327.4
4. Surface tension (dyne/cm): 31.8
5. Polarizability (10-24cm3): 14.76
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 5
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 162
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Basic properties: This product solution turns red when exposed to ferric chloride
Storage method
Storage: Store in a dry and sealed room at room temperature, away from contact with moist air.
Synthesis method
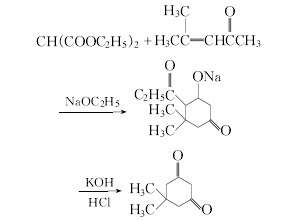
Slowly add diethyl malonate to the ethanol solution of sodium alkoxide at room temperature and stir evenly, then slowly add isopropyl acetone while cooling with water. , then heated to 50°C and maintained for 2 hours. Most of the ethanol was evaporated under reduced pressure, cooled, and filtered. The filtered solid was dissolved in 15% potassium hydroxide.Solution, constant temperature 60℃, stir the reaction thoroughly to obtain 5,5-dimethyl-1,3-cyclohexanedione. The obtained crude product can be adjusted to pH=4.5 with 4mol/L dilute hydrochloric acid at 30℃, and filtered to dryness. Recrystallize in ethanol and purify.

Purpose
This product can form insoluble condensates with aldehydes, while ketones do not have this feature, so it is used to separate or test aldehydes. This product can also be used as a catalyst for pyridine and a chromatographic analysis reagent.