1,3-Dibromopropane 1,3-Dibromopropane
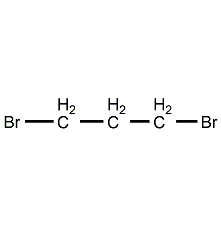
Structural formula
| Business number | 02ZD |
|---|---|
| Molecular formula | C3H6Br2 |
| Molecular weight | 201.89 |
| label |
Trimethylene dibromide, Trimethyl bromide, trimethylene bromide, Dibromo-1,3 propane, aliphatic compounds, Halogenated hydrocarbon solvents |
Numbering system
CAS number:109-64-8
MDL number:MFCD00000255
EINECS number:203-690-3
RTECS number:TX8575000
BRN number:635662
PubChem number:24847685
Physical property data
1. Properties: colorless or light yellow liquid, odorless, sweet taste.
2. Density (g/mL, 20/4℃): 1.982
3. Melting point (ºC): -34.2
4. Boiling point (ºC ,167.3): 165
5. Boiling point (ºC, 2.67kPa): 59~63
6. Boiling point (ºC, 1.33kPa): 55.6
7. Refractive index (15ºC): 1.5249
8. Flash point (ºC): 54
9. Solubility: Soluble in ether, acetone and chloroform, insoluble in water.
Toxicological data
1. Acute toxicity: Rat oral LD50: 315mg/kg2. Harmful by inhalation and oral administration.
Ecological data
This substance is harmful to water bodies and can poison fish.
Molecular structure data
1. Molar refractive index: 31.41
2. Molar volume (cm3/mol): 104.4
3. Isotonic specific volume (90.2K ): 255.7
4. Surface tension (dyne/cm): 36.0
5. Polarizability (10-24cm3): 12.45
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 12.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
No contact with oxides. Easy to absorb moisture, avoid contact with skin.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. They should be stored separately from oxidants and should not be mixed.��. It should not be stored for a long time to avoid deterioration. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of propylene glycol and hydrobromic acid. First add concentrated sulfuric acid and 1,3-propanediol to hydrobromic acid, then slowly drop in concentrated sulfuric acid, reflux on a sand bath for 7 hours, then evaporate the crude product, wash the crude product once with water, and use 10% sodium thiosulfate Wash the solution 2-3 times, wash twice with 10% sodium carbonate, separate the aqueous layer, and dry with anhydrous calcium chloride. Fractionate under normal pressure and collect the 159-168°C fraction to obtain the finished product.
2. Preparation method:
![]()
In a reaction flask equipped with a stirrer, reflux condenser, and dropping funnel, add 500g (340mL) of 48% hydrobromic acid, cool in an ice-water bath, and slowly add 80mL of concentrated sulfuric acid while stirring. Add 91g of newly distilled 1,3-propanediol (2) dropwise (bp210~215℃), and then add 130mL of concentrated sulfuric acid while stirring. After adding, heat to reflux for 3.5 hours. Change to a distillation unit for distillation. Until there is almost no oil dripping out. The distillate was distilled, and the fraction between 162 and 165°C was collected to obtain 220g of 1,3-dibromopropane with a yield of 91%. [1]
Purpose
Used in organic synthesis. Also used in the synthesis of melatonin.