3-Penten-2-one
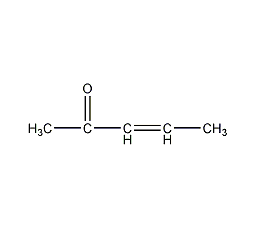
Structural formula
| Business number | 06RW |
|---|---|
| Molecular formula | C5H8O |
| Molecular weight | 84.11 |
| label |
Ethylene Acetone, Methyl propenyl ketone, Ethylidene acetone, Methyl 1-propenyl ketone |
Numbering system
CAS number:625-33-2
MDL number:MFCD00009290
EINECS number:210-888-3
RTECS number:SB3850000
BRN number:1633505
PubChem number:24848542
Physical property data
1. Properties: colorless flammable liquid.
2. Density (g/mL, 25/4℃): 0.862
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): 121~124
6. Boiling point (ºC, 5.2kPa): Undetermined Confirm
7. Refractive index (n20D): 1.437
8. Flash Point (ºC): 21
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol) : Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol /water) logarithmic value of the distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V) : Undetermined
19. Solubility: Soluble in water, ether, acetone.
Toxicological data
1. Acute toxicity: Rat (oral) LD50: 3,200 mg/kg
Rat (inhalation) LCLo: 250 ppm/4H
Rat (skin) LD50: 5 00μg/kg
Since the LD50 of table salt is 3,000 mg/kg, the acute toxicity of BPA is the same as that of table salt.
Ecological data
Do not allow large quantities of products that are slightly harmful to water to come into contact with groundwater, waterways or sewage systems. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 25.30
2. Molar volume (cm3/mol): 101.8
3. Isotonic specific volume (90.2K ): 223.3
4. Surface tension��dyne/cm): 23.1
5. Polarizability (10-24cm3): 10.02
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 0.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Topological molecular polar surface area (TPSA): 17.1
6. Number of heavy atoms: 6
7. Surface charge: 0
8. Complexity: 72
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters : 0
11. Uncertain number of atomic stereocenters: 0
12. Determined number of chemical bond stereocenters: 0
13. Uncertain chemical bond formation Number of structural centers: 0
14. Number of covalent bond units: 1
Properties and stability
1. Stay away from oxides.
2. Exist in flue-cured tobacco leaves and mainstream smoke.
Storage method
Store in an airtight container in a cool, dry place. Store away from oxidizing agents. Storage temperature 4ºC
Synthesis method
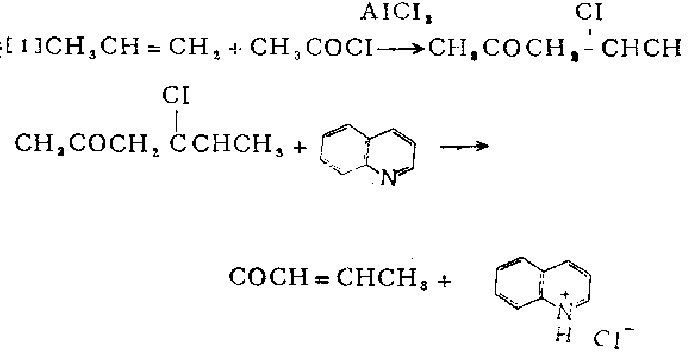
1. Found in cranberries, bilberries, fried peanuts, and potato chips. It is produced by the condensation and dehydration of acetone and acetaldehyde under alkaline conditions, or the reaction of propylene and acetyl chloride in the presence of aluminum trichloride.
2. Note that this reaction must be carried out in a fume hood.
The 2-liter three-neck flask is equipped with an effective mechanical stirrer, a gas input pipe (extending almost to the bottom of the bottle), and a high-efficiency reflux condenser with a calcium chloride drying tube. After drying the instrument in an oven, 800 ml of dry methylene chloride and 157 g (142 ml, 2.0 mol) of acetyl chloride were placed in the bottle. Stir the solution and add 320 grams (2.4} mol) powdered anhydrous aluminum chloride portionwise within ].;} minutes. Once the addition is complete, a stream of propylene gas is introduced into the continuously stirred reaction solution at a rate sufficient to provide gentle reflux. Continue gas flow until no more heat is released and reflux ceases (10-30 hours). At this point, the bottle is nearly full. When the stirring stops, the contents separate into two layers. Carefully pour the contents of the bottle onto 1.5 kg of ice. Separate the upper organic layer. The aqueous layer was extracted with 3 portions}0} ml of methylene chloride. Combine the organic solutions, wash with 50 ml of water, and dry over anhydrous magnesium sulfate. Place the obtained dark brown solution in a domestic flask equipped with a thermometer, a magnetic stirrer and a vacuum tin evaporation device, and use a heating mantle to provide heat. While maintaining the mixture at 0°C, the mixture was evaporated under reduced pressure with a water pump. Most of what evaporates from the mixture is methylene chloride and volatile hydrocarbons. After most of the solvent is evaporated, a 1-liter round-bottomed flask cooled in a dry ice-isopropyl alcohol bath is connected to the tin evaporation device as a receiver. , reduce the pressure to <lmm. Use a heating mantle to provide heat to slowly raise the viscous liquid in the radium evaporator bottle from 0°C to 45°C within 90 minutes. At this time, volatile products (including methylene chloride, low molecular weight hydrocarbons, 4-chloropentene-2-one and 3-pentene-2-one) are distilled out. 400 to 500 grams of light green distillate was obtained, mixed with 256 grams (1i mol) of sodium chloride, and then heated to boiling. In order to remove residual methylene chloride and other low-boiling substances, the mixture is evaporated until the temperature of the distillate rises to 110-120°C. The remaining solution is refluxed for 30 minutes, then cooled, and used with the previously evaporated distillate and Dilute with 200 ml of pentane, and wash the resulting solution with several portions of 250 ml of 10% hydrochloric acid aqueous solution until the wash becomes acidic. Combine the aqueous solutions and acidify. Shake with 3 parts of 100 mg pentane. The organic solutions were combined, washed with 50 ml of saturated sodium bicarbonate solution, and then dried over anhydrous magnesium sulfate. The obtained organic solution is used in a 30 cm long pine needle-shaped fractionation column to collect the 119-124°C fraction. The product weighs 42-63
g (25-37% ). Purity is 86-92%. If a higher purity product is required, a 60 cm spinneret column can be used for distillation. Since distillation may be accompanied by partial isomerization of a, β-unsaturated ketones into iso-β, γ-isomers with lower boiling points, the products obtained by fractionation should be subjected to acid-catalyzed equilibrium treatment. A typical purification method is to mix 79.4 grams of a mixture of pentene-2-one isomers with a boiling point of 117-119 with 400 mg of p-toluenesulfonic acid and reflux for 30 minutes. The resulting mixture was diluted with 100 ml of diethyl ether, washed with 50 ml of saturated aqueous sodium bicarbonate solution, and dried over anhydrous magnesium sulfate.

3. Tobacco: FC, 40.
Purpose
Used as synthetic reagents and cigarette flavorings. Robinson ring-enlarging reaction.