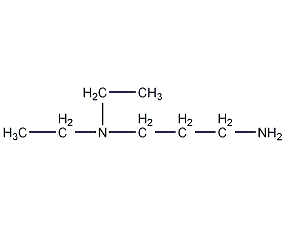
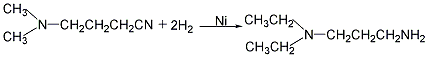N,N-Dimethyl-1,3-propanediamine N,N-Dimethyl-1,3-propanediamine
Structural formula
| Business number | 02Z5 |
|---|---|
| Molecular formula | C5H14N2 |
| Molecular weight | 102 |
| label |
N,N-dimethyltrimethylenediamine, dimethylaminopropylamine, 3-dimethylaminopropylamine, N,N-dimethyl-trimethylene diamine, dimethylamino propylamine |
Numbering system
CAS number:109-55-7
MDL number:MFCD00008216
EINECS number:203-680-9
RTECS number:TX7525000
BRN number:605293
PubChem number:24864543
Physical property data
1. Properties: colorless liquid with ammonia smell. [1]
2. Melting point (℃): <-70[2]
3. Boiling point (℃) : 123[3]
4. Relative density (water=1): 0.81 (30℃)[4]
5. Relative vapor density (air=1): 3.52[5]
6. Saturated vapor pressure (kPa): 1.33 (30℃)[6]
7. Octanol/water partition coefficient: -0.45[7]
8. Flash point (℃): 37.78 (OC )[8]
9. Solubility: miscible with water and soluble in organic solvents. [9]
Toxicological data
1. Skin/eye irritation
Open irritation test: rabbit, skin contact: 100μg/24H;
Standard Draize test: rabbit, eye contact: 5mg, Severity of reaction: Moderate.
2. Acute toxicity:
Oral LD50 in rats: 1870mg/kg; LD50 in rabbit skin contact: 600μL/kg;
3 .Acute toxicity[10] LC50: 1000mg/m3 (rat inhalation, 2h)
4. Irritation No data available
5. Subacute and chronic toxicity[11] Rabbits and dogs, 60 mg/m3, 5 hours a day for 30 days, can cause eye and respiratory tract irritation and weight loss.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[12] This substance may be harmful to the environment and is harmful to the environment. Bodies of water should be given special attention.
Molecular structure data
1. Molar refractive index: 32.46
2. Molar volume (cm3/mol): 122.0
3. Isotonic specific volume (90.2K ): 285.7
4. Surface tension (dyne/cm): 30.1
5. Polarizability: 12.87
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.3
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 29.3
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 35.1
p>
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is more flammable when exposed to open flames, high temperatures, and oxidants; high heat releases toxic gases. Highly toxic. Production equipment must be sealed to prevent running, popping, dripping and leaking. Operators should wear protective equipment to avoid direct contact with this product.
2. This product is highly toxic. This product can cause serious damage if swallowed or inhaled. Strongly irritating to skin. Rat oral administration: LD501870mg/kg. Production equipment should be sealed to prevent running, emitting, dripping and leaking. The operating area should be well ventilated, and operators should wear protective gear.
3. Stability[13] Stable
4. Incompatible substances[14] Strong oxidizing agent
5. Conditions to avoid contact[15] Contact with air
6. Aggregation hazards[16] No aggregation
Storage method
1. Storage precautions[17] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. The packaging must be sealed and must not come into contact with air. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. This product is packed in galvanized iron barrel, 170kg/barrel. Or in glass bottles, each bottle has a net weight of 0.8kg, and every 10 bottles is packed in a wooden box. Store in a cool and ventilated place. Storage period is one year. Inflammable materials, fire is strictly prohibited. Loading, unloading and fire prevention storage and transportation shall be carried out according to the regulations of flammable and toxic chemicals.
Synthesis method
Use dimethylaminopropionitrile [1738-25-6] as raw material, hydrogenate and methanol in the presence of Ni-Al catalyst, and then filter and distill to obtain 3-dimethylaminopropylamine finished product. The purity of the obtained product can reach more than 99%, and each ton of product consumes 1150kg of dimethylaminopropionitrile.
(1) First, N, N-dimethylaminopropionitrile is synthesized from acrylonitrile and dimethylamine as raw materials, and then obtained by hydrogenation.
![]()
1. Dimethylhydropropyl Synthesis of nitrile: Slowly add 33% dimethylamine aqueous solution (0.4mol) dropwise to 21.2g (0.4mol) acrylonitrile. During the dripping process, the temperature of the reaction solution is controlled at 10-15°C. The dripping time is about 2-3h. Stir at the same temperature for 3-4 hours and let stand overnight. Distill under reduced pressure at 5.33KPa, collect the 82-84°C fraction, and obtain 36.4 dimethylaminopropionitrile intermediate product.
2. Synthesis of dimethylpropylenediamine Add 300kg dimethylaminopropionitrile and 1.2g catalyst into the autoclave, seal it, replace the air in the autoclave with nitrogen three times, and then press in about 40g of liquid Ammonia, add air, react at 60-70℃, 2.0-3.0MPa for 1.5h. The crude product obtained from the reaction is distilled to obtain the finished product.
![]()
(2) In clean 500ml In the beaker, add 20g of acrylamide and 25ml of epichlorohydrin respectively, and add NAOH to adjust the pH value of the reaction medium. Stir the reaction at 40°C for 12 hours. After cooling, 80 ml of 33% dimethylamine aqueous solution is added dropwise in a sealed manner, dried with anhydrous sodium sulfate at room temperature for several times, and then continuously extracted with chloroform several times to obtain a light yellow oily liquid. The yield is about 82%. After drying, the product was dissolved in a small amount of water, and sodium tetraphenylborate solution was added dropwise. A large amount of white precipitate immediately formed, which can be confirmed to be DMAPA with a positive nitrogen cation structure.
Purpose
1. Used as an organic synthesis intermediate to prepare dyes, ion exchange resins, epoxy resin curing agents, oils and cyanide-free electroplating additives, fiber and leather treatment agents and fungicides, etc. The molecule of this product contains both primary amine groups and tertiary amine groups. It is used as an epoxy resin curing agent and has the functions of both curing agent and accelerator. Mainly used for laminated products, cast products and adhesives, etc. The general dosage is 2-6 parts.
2. Used as a curing agent for epoxy resin. The molecule of this product contains both primary amine groups and tertiary amine groups. It is used as an epoxy resin curing agent and has the functions of accelerator and accelerator. It is mainly used for laminates, cast products, adhesives, etc. The general dosage is 2 to 6 parts. This product is also used as an organic synthesis intermediate to prepare dyes, ion exchange resins and galvanizing additives.
3. Used as epoxy resin curing agent, the reference dosage is 5 to 10 parts by mass, the curing conditions are 60°C/4h+120°C/1h, and the thermal deformation temperature of the cured product is 78 to 94°C. It can also be used as a cyanide-free electroplating additive, a leather and fiber treatment agent, and an intermediate for preparing pesticides, dyes, ion exchange resins, etc. React with coconut oil ester, methyl oleate and methyl stearate to produce corresponding alkylamidopropyl betaine products, which can be used for fabric softening and household cleaning agents.
4. Used in organic synthesis and as epoxy resin curing agent. [18]