1,3-Propanediamine 1,3-Propanediamine
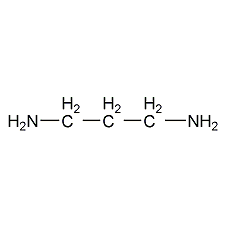
Structural formula
| Business number | 02ZQ |
|---|---|
| Molecular formula | C3H10N2 |
| Molecular weight | 74.12 |
| label |
1,3-diaminopropane, propylenediamine, H2NCH2CH2CH2NH2, Propane-1,3-diamine, 1,3-Diaminopropane, purifier, Lubricant additives, Nitrogen-containing compound solvent |
Numbering system
CAS number:109-76-2
MDL number:MFCD00008228
EINECS number:203-702-7
RTECS number:TX6825000
BRN number:605277
PubChem number:24859902
Physical property data
1. Properties: colorless liquid, hygroscopic, with an ammonia smell. [1]
2. Melting point (℃): -12[2]
3. Boiling point (℃): 139.7[3]
4. Relative density (water = 1): 0.89[4]
5. Relative vapor Density (air=1): 2.5[5]
6. Saturated vapor pressure (kPa): <1.07 (20℃)[6]
7. Critical temperature (℃): 333.4[7]
8. Critical pressure (MPa): 5.12[8] sup>
9. Flash point (℃): 48.9[9]
10. Ignition temperature (℃): 350[10 ]
11. Explosion upper limit (%): 15.2[11]
12. Explosion lower limit (%): 2.8 [12]
13. Solubility: easily soluble in water, soluble in methanol and ether. [13]
14. Refractive index (20ºC): 1.4555
15. Viscosity (mPa·s, 20ºC): 2.0
Toxicological data
1. Acute toxicity[14]
LD50: 312mg/kg (rat oral); 0.2ml (178mg)/kg (Rabbit transdermal)
2. Irritation [15]
Rabbit transdermal: 50 mg, severe irritation ( open stimulus test).
Rabbit eye: 1mg, severe irritation.
Ecological data
1. Ecotoxicity[16] LC50: 13mg/L (96h) (fish)
2. Biodegradation Properties No data available
3. Non-biodegradability No data available
Molecular structure data
1. Molar refractive index: 23.01
2. Molar volume (cm3/mol): 85.4
3. Isotonic specific volume (90.2K ): 209.5
4. Surface tension (dyne/cm): 36.2
5. Polarizability (10-24cm3):9.12
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 52
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 12.4
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain atoms Number of stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Its chemical properties are polar compounds, which can form hydrogen bonds, alkylation and acylation, and can form corresponding salts with both inorganic and organic acids.
2. Stability[17] Stable
3. Incompatible substances[18] Acids, acid chlorides, acid anhydrides, strong oxidants, carbon dioxide
4. Conditions to avoid contact [19] Heating
5. Polymerization hazard[20] No polymerization
Storage method
Storage Precautions[21] Stored in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 35℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Mix 1 part of dibromopropane with 8-9 parts of saturated ammonia and ethanol solution at 0°C, and leave it for 3-4 days. Evaporate to dryness, add alkali to the residue and then distill. The distillate was neutralized with hydrochloric acid. Evaporate to concentrate, cool, and dry the concentrated solution to crystallize the hydrochloride. Use alkali to decompose the hydrochloride and distill out 1,3-propanediamine.
Purpose
1. Used as organic synthesis intermediates and solvents. It is used in the synthesis of medicines and pesticides. It is an auxiliary raw material in the papermaking, textile and leather industries. It is also used in the synthesis of epoxy resin curing agents, synthetic fuel oil and lubricating oil additives.
2. Used as organic synthesis intermediates and solvents. [22]