2,3-Dichloro-5,6-dicyano-1,4-benzoquinone 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
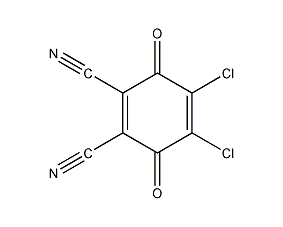
Structural formula
| Business number | 01UR |
|---|---|
| Molecular formula | C8Cl2N2O2 |
| Molecular weight | 227.00 |
| label |
Diaminodicyanoquinone, 2,3-Dichloro-5,6-dicyanobenzoquinone, 2,3-Dichloro-5,6-dicyanobenzoquinone, 4,5-Dichloro-3,6-dioxo-1,4-cyclohexadiene-1,2-dicarbonitrile, DDQ, dehydrogenation reagent, oxidizing agent |
Numbering system
CAS number:84-58-2
MDL number:MFCD00001593
EINECS number:201-542-2
RTECS number:GU4825000
BRN number:747939
PubChem number:24893996
Physical property data
1. Properties: yellow solid
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air =1): Undetermined
4. Melting point (ºC): 213~216
5. Boiling point (ºC, normal pressure): Undetermined
6 . Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9 . Specific rotation (º): Not determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
<p15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Upper explosion limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in ethyl acetate Ester and THF, soluble in dichloromethane, benzene, dioxane and acetic acid, slightly soluble in water.
Toxicological data
1. Acute toxicity:
Mouse abdominal LD50: 31mg/kg;
Mouse intravenous LD50: 13mg/kg;
2. Teratology
Salmonella: 180nmol/plate;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 45.73
2. Molar volume (cm3/mol): 133.4
3. Isotonic specific volume (90.2K): 388.0
4. Surface tension (dyne/cm): 71.4
5. Polarizability (10-24cm3): 18.13
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 81.7
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 464
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. ConfirmThe number of stereocenters of definite chemical bonds: 0
14. The number of stereocenters of uncertain chemical bonds: 0
15. The number of covalent bond units: 1
Properties and stability
It has certain stability to air, but will release HCN when in contact with water. It must be operated and used in a water-free environment protected by inert gas.
Storage method
Store sealed in a cool, dry place.
Synthesis method
1. Stir 2,3-dicyanohydroquinone and hydrochloric acid into a slurry, slowly add 70% nitric acid at about 35°C, and continue stirring for 1 hour. Filter, wash with carbon tetrachloride, and dry to obtain DDQ with a yield of 90%.
2.Add 10 grams (0.062 mol) 2 into a three-neck flask equipped with a stirrer, reflux condenser and gas introduction tube , 3-dicyanide and 100 ml of glacial acetic acid, heated to slightly boiling. Dry chlorine gas was introduced at a rate of 0.2 grams per minute. After 1 hour, crystals began to precipitate in the refluxed reaction solution. After the reaction was continued for 30 minutes, the flow of chlorine was stopped. Cool to room temperature, filter out the solid, wash with 50 ml of chloroform, and dry to obtain 6.4 g (44%) of white crystals. It crystallizes from water-ethanol to obtain needle-like crystals, which show no change when heated to 300°C.
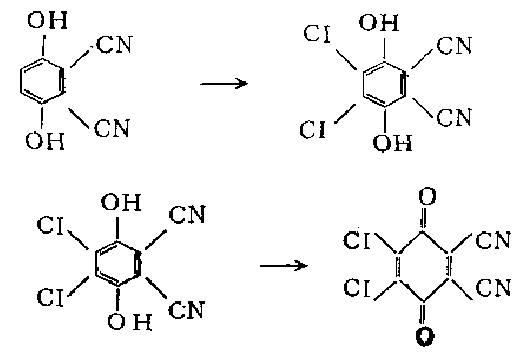
3. Add 10 grams (0.044 mol) of 2,3-dichloro-5,6-dihydrohydrogen, 40 ml of ethanol and 110 ml of 5 in a stoppered Erlenmeyer flask. % hydrochloric acid, shake to form a suspension. Add 220 ml of benzene and 44 g (0.184 mol) of lead dioxide, and shake vigorously for 5 minutes. The emulsion was filtered on a Buchner funnel filled with a layer of diatomaceous earth, and the residue was washed with benzene. Combine the filtrate, use a separatory funnel to separate the aqueous layer, wash the organic layer twice with a small amount of water, dry , concentrate to precipitate red crystals, add petroleum ether (30-60℃) Complete precipitation. Filter, wash with petroleum ether, and dry to obtain 8.3 grams (83%) of the product as yellow crystals.
Purpose
1. Oxidizing agent for the synthesis of steroid compounds, used in the production of family planning drugs chlormadinone and trienogestrol.
2. Commonly used dehydrogenation reagents. It is widely used in reactions such as dehydrogenation, oxidation, aromatization and cycloaddition.
3.DDQ is a highly active oxidant [1], which can be used in dehydrogenation reactions to prepare aromatic compounds and α,β -Unsaturated carbonyl compounds. In addition, DDQ can also oxidize active methylene and hydroxyl groups to obtain the corresponding carbonyl compounds. Phenolic compounds are very sensitive to DDQ.
Under the action of DDQ, the transfer process of substrate hydrogen ions to DDQ can occur, thereby realizing the dehydrogenation reaction of the substrate, and DDQ is converted into diphenol. Therefore, the dehydrogenation reaction is related to the stability of the carbocation formed initially in the reaction, and is particularly effective for compounds with ortho-stabilizing functional groups. Inert hydrocarbons are very stable to DDQ, while substrates containing alkenyl or aryl groups can be oxidized by DDQ to obtain conjugated products (Formula 1, Formula 2)[2,3].
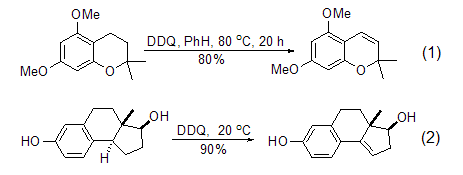
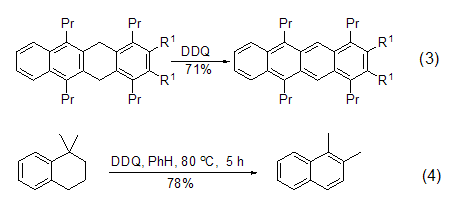
DDQ is a very effective aromatic Kylating reagents are often used in the dehydrogenation reaction of simple or complex hydrogenated aromatic carbocyclic compounds to obtain conjugated aromatic compounds (formula 3)[4]. The oxidative dehydrogenation reaction of quinone compounds usually does not cause skeletal rearrangement, but 1,1-dimethyltetrahydronaphthalene can easily undergo aromatization reaction accompanied by 1,2-hydrogen transfer under the action of DDQ (Equation 4) [5].
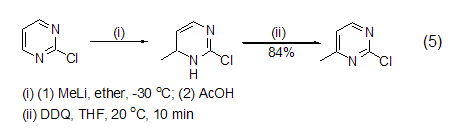
In addition, hydrogenated aromatic heterocyclic compounds It can also be oxidatively dehydrogenated by DDQ to obtain heterocyclic aromatic hydrocarbons such as pyrrole, pyrazole, triazole, pyridine, pyrazine, indole, quinoline, furan, thiophene, isothiophene (Formula 5)[6].
In addition to obtaining neutral aromatic compounds In addition, DDQ can also be used to prepare salts of stable aromatic cations, such as oxidation in the presence of perchloric acid to obtain triphenylcyclopropane cations (formula 6)[7].
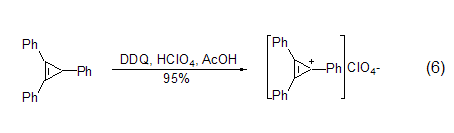
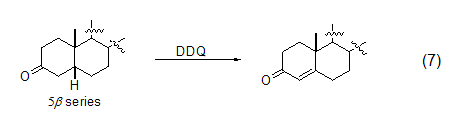
Like other quinone compounds, DDQ is also an effective oxidizing agent for the preparation of α,β-unsaturated carbonyl compounds. This reaction with carbonyl substrates has been extended to 3-ketosteroid chemistry. The regioselectivity of the dehydrogenation reaction depends on the stereochemistry of the substrate (Equation 7)[8].
However, DDQ-induced carbonyl compounds Dehydrogenation reactions are still mainly limited to carbonyl substrates that are easily enolized. Therefore, the dehydrogenation reactions of esters and amide compounds often require more harsh reaction conditions. For example, adding the silylation reagent BSTFA will help the dehydration of lactams. Hydrogen reaction (Formula 8)[9].
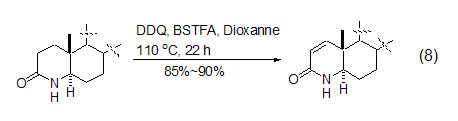
DDQ can also be used in alcohols The oxidation reaction of the compound oxidizes the hydroxyl group to the carbonyl group. Saturated alcohols are stable to DDQ in the absence of light, while some sterically forbidden secondary alcohols are easily oxidized by DDQ to the corresponding ketones (Formula 9)[10].
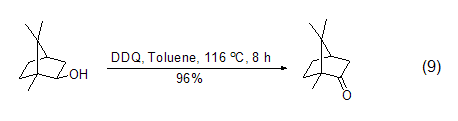
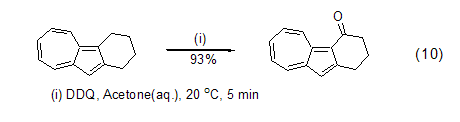
DDQ can also be used to oxidize benzyl The alkyl group of the base compound is used to obtain a benzyl carbocation intermediate, which can then react with other nucleophiles to obtain benzyl ketone or benzyl substituted compounds (formula 10)[11].
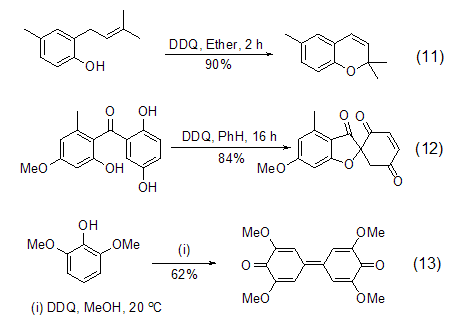
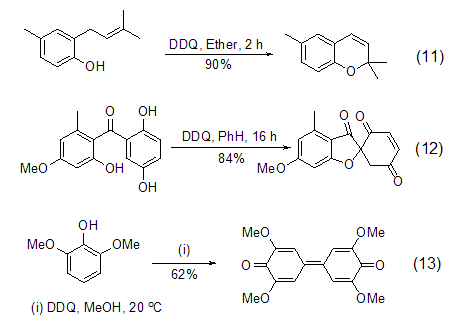
DDQ is an oxidation agent for phenolic compounds An ideal reagent that can realize intramolecular ring-forming reactions (Formula 11, Formula 12) [12] and coupling reactions (Formula 13) [13] of phenolic compounds.
The corresponding ketone (Formula 9)[10].

DDQ can also be used to oxidize benzyl The alkyl group of the base compound is used to obtain a benzyl carbocation intermediate, which can then react with other nucleophiles to obtain benzyl ketone or benzyl substituted compounds (formula 10)[11].

DDQ is an oxidation agent for phenolic compounds An ideal reagent that can realize intramolecular ring-forming reactions (Formula 11, Formula 12) [12] and coupling reactions (Formula 13) [13] of phenolic compounds.
