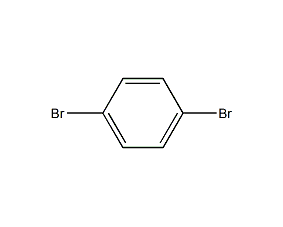
1,4-Dibromobenzene 1,4-Dibromobenzene
Structural formula
| Business number | 02T9 |
|---|---|
| Molecular formula | C6H4Br2 |
| Molecular weight | 235.90 |
| label |
1,4-Dibromobenzene, p-dibromobenzene, Dibromobenzene, 1,4-Dibromobenzene, Para-Dibromobenzene, P-Benzene Dibromide, P-Dibromobenzene, 1,4-Dibrombenzol, 1,4-dibromo-benzen, Benzene, p-dibromo-, Benzene,1,4-dibromo- |
Numbering system
CAS number:106-37-6
MDL number:MFCD00000089
EINECS number:203-390-2
RTECS number:CZ1791000
BRN number:1904543
PubChem ID:None
Physical property data
1. Properties: off-white crystal.
2. Density (g/mL, 25℃): 1.8410
3. Relative density (20℃, 4℃): 1.26117
4. Melting point (ºC): 87.3
5. Boiling point (ºC, normal pressure): 218.5
6. Relative density (25℃, 4℃): 1.964199.6
7. Refractive index (D20): 1.5742
8. Gas phase Standard entropy (J·mol-1·K-1): 359.87
9. Gas phase standard hot melt (J·mol– 1·K-1): 117.64
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor Pressure (mmHg, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 20ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
p>
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil-water (octanol/water) partition coefficient Logarithmic value of p>
19. Solubility: Insoluble in water, easily soluble in benzene and chloroform.
Toxicological data
1. Acute toxicity: rat oral LD50: 3120mg/kg; mouse abdominal LD50: 1891mg/kg;
2. Other multiple dose toxicity: rat oral TDLo: 1800mg/ kg/90D-I;
Ecological data
This substance may be harmful to the environment. Special attention should be paid to the pollution of water bodies, especially the occurrence of bioaccumulation in aquatic organisms.
Molecular structure data
1. Molar refractive index: 41.63
2. Molar volume (cm3/mol): 121.8
3. Isotonic specific volume (90.2K): 308.2
4. Surface tension (dyne/cm): 41.0
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 16.50
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 54.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The storage temperature should not exceed 30℃. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
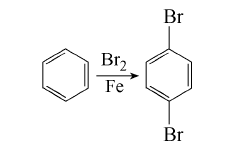
Add 2g iron filings to 1mol benzene, slowly add bromine dropwise, usually the reaction will start after 15 minutes (slow boiling), control the rate of adding bromine to keep the reactant slightly boil. After the bromine is added, heat at 20~30°C for 1 hour, and then heat at 60°C for 45 minutes until the brown vapor of bromine disappears. Filter out the iron filings, wash with water and then steam. The aqueous layer of the steamed bromobenzene in the first part is separated, dried with calcium chloride and then distilled. The fraction collected at 150~170℃ is mainly bromobenzene. The residue after distillation was poured into a porcelain dish while hot, condensed and combined with the p-dibromobenzene obtained by steam distillation. After drying, activated carbon was added and recrystallized with methanol to obtain 24g of p-dibromobenzene, with a yield of 10%. 
Purpose
Used in organic synthesis, dye intermediates.