2,4-Dichloro-1-(dichloromethyl)benzene
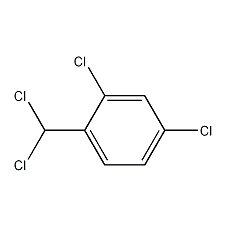

Structural formula
| Business number | 03PF |
|---|---|
| Molecular formula | C7H4Cl4 |
| Molecular weight | 229.92 |
| label |
2,4-Dichlorobenzylidene dichloride, α,α,2,4-Tetrachlorotoluene, aromatic compounds |
Numbering system
CAS number:134-25-8
MDL number:None
EINECS number:205-134-5
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
None
Toxicological data
None
Ecological data
None
Molecular structure data
Molecular property data:
1、 Molar refractive index:50.66
2, Molar volume(m 3/mol):153.1
3, Isotonic specific volume(90.2K):391.3
4, Surface tension(3.0 dyne/cm SPAN>): 42.6
5, Polarizability( 0.5 10-24cm3): 20.08
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 126
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
The preparation method is to add 2,4-dichlorotoluene and a small amount of PCl3 as a catalyst into a reaction kettle, heat it to 120°C, and introduce chlorine gas under light conditions to perform a chlorination reaction. When a sample is taken and analyzed by GC, 2,4 -The conversion rate of dichlorotoluene ≥99% is the end point of the chlorination reaction. The obtained chlorinated products include 0.62% of 2,4-dichlorobenzyl chloride, 85.18% of 2,4-dichlorobenzylidene dichloride, and 13.8% of 2,4-dichlorotrichloromethylbenzene, which are separated after post-processing. 2,4-Dichlorobenzylidene dichloride.
Purpose
2,4-Dichlorobenzylidene dichloride is an intermediate for the preparation of 2,4-dichlorobenzaldehyde and can be used to produce fungicides such as ethyl alcohol.
20.08
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 126
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
The preparation method is to add 2,4-dichlorotoluene and a small amount of PCl3 as a catalyst into a reaction kettle, heat it to 120°C, and introduce chlorine gas under light conditions to perform a chlorination reaction. When a sample is taken and analyzed by GC, 2,4 -The conversion rate of dichlorotoluene ≥99% is the end point of the chlorination reaction. The obtained chlorinated products include 0.62% of 2,4-dichlorobenzyl chloride, 85.18% of 2,4-dichlorobenzylidene dichloride, and 13.8% of 2,4-dichlorotrichloromethylbenzene, which are separated after post-processing. 2,4-Dichlorobenzylidene dichloride.
Purpose
2,4-Dichlorobenzylidene dichloride is an intermediate for the preparation of 2,4-dichlorobenzaldehyde and can be used to produce fungicides such as ethyl alcohol.