2,4-Dichlorophenol 2,4-Dichlorophenol
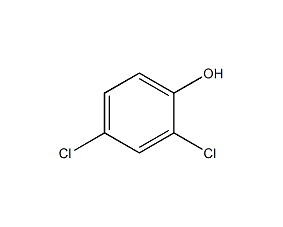

Structural formula
| Business number | 03CX |
|---|---|
| Molecular formula | C6H4Cl2O |
| Molecular weight | 163.00 |
| label |
2,4-Dichlorophenol, 1-hydroxy-2,4-dichlorobenzene, 4,6-Dichlorophenol, 1-Hydroxy-2,4-dichlorobenzene, Pesticide intermediates; phenols, aromatic alcohols and their derivatives |
Numbering system
CAS number:120-83-2
MDL number:MFCD00002169
EINECS number:204-429-6
RTECS number:SK8575000
BRN number:742467
PubChem number:24849305
Physical property data
1. Properties: white needle-like crystals
2. Relative density (60ºC): 1.383
3. Flash point (ºC): 113
4. Melting point (ºC): 42~43
5. Boiling point (ºC): 210
6. Water solubility (g/L, 20 ºC): 4.5
7. Solubility: Hardly soluble in water, easily soluble in ethanol, ether, chloroform, benzene and other organic solvents.
8. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -165.1
Toxicological data
It is irritating to human skin and eyes, and its dust is also irritating to human respiratory system. It is a less toxic substance. The acute oral LD50 in mice is 0.58g/kg. It is corrosive and can cause burns.
Ecological data
Toxic to aquatic life. May cause adverse consequences for the aquatic environment.
Molecular structure data
1. Molar refractive index: 37.92
2. Molar volume (cm3/mol): 111.7
3. Isotonic specific volume (90.2K ): 294.0
4. Surface tension (dyne/cm): 47.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability 15.03
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 97.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. White needle-like crystals. Can evaporate with water vapor. Soluble in ethanol, ether, benzene and carbon tetrachloride, slightly soluble in water. Strongly irritating to tissues.
2. Easily volatile and highly corrosive, it can burn skin and irritate eyes and skin. Severe poisoning can cause anemia and various neurological symptoms. Those with skin allergies can cause dermatitis that is difficult to cure. The workshop should be well ventilated and the equipment should be sealed. Masks, glasses and rubber gloves should be worn during operation. If it accidentally splashes on the skin, immediately scrub it with alcohol or rinse it with dilute alkaline water. If it has been ingested, gastric lavage should be performed immediately with warm water and magnesium oxide (30g/L). If it splashes on clothes, change clothes and take a shower immediately to prevent it from penetrating into the skin.
Storage method
Save in a cool and dark place.
Packed in iron drums. This product is flammable and should be kept away from fire sources and stored in a cool, dry and ventilated place. When a 2,4-dichloro(phenyl)phenol fire occurs, use water, yellow sand, and foam carbon dioxide to extinguish the fire.
Synthesis method
Using phenol catalytic chlorination method to produce 2,4-dichlorophenol. Pump the molten phenol into the chlorination tank and absorption tank, heat it to 50~60ºC, and introduce chlorine gas. The chlorine gas first enters the chlorination tank, and then enters the absorption tank in series. The tail gas is passed into the graphite film absorption tower, and the hydrogen chloride in the tail gas is absorbed with water. . The cooling water in the jacket and coil pipe of the reaction tank removes the reaction heat. When the relative density of the material in the chlorination tank reaches 1.402~1.405 (40ºC), it is the end point of chlorination. The flow of chlorine gas will stop and 2,4-dichlorophenol will be released from the chlorination tank. When the next batch of chlorination is chlorinated, the original absorption tank will be used as the chlorination tank, fresh chlorine gas will be introduced first, and the raw material phenol will be pumped into the original chlorination tank to be used as the absorption tank.
Purpose
Used as solvent, pesticide and pharmaceutical intermediate. Used to synthesize herbicidal ether; 2,4-D; EPBP; Dukesan; fentanyl and the drug thiobisdichlorophenol.