1,4-Dihydroxyanthraquinone 1,4-Dihydroxyanthraquinone

Structural formula
| Business number | 01SC |
|---|---|
| Molecular formula | C14H8O4 |
| Molecular weight | 240.21 |
| label |
Quezain, quinone, Aromatic aldehydes, ketones and their derivatives |
Numbering system
CAS number:81-64-1
MDL number:MFCD00001209
EINECS number:201-368-7
RTECS number:CB6600000
BRN number:1914036
PubChem number:24863338
Physical property data
1. Properties: What is precipitated from acetic acid and ether is orange crystal, while what is precipitated from alcohol, benzene and toluene is dark red needle crystal.
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 200~202 (precipitated from acetic acid), 196 (precipitated from ethanol).
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition Combustion temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Uncertain
17. The upper limit of explosion (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Slightly soluble in water, soluble in concentrated sulfuric acid, sodium hydroxide solution, chlorobenzene, toluene, xylene , Dichlorobenzene, it is red when soluble in alcohol, brown and yellow fluorescent when soluble in ether, purple when soluble in alkali and ammonia. It forms a black precipitate when exposed to carbon dioxide, and 1g can be dissolved in 13g of boiling glacial acetic acid. Can be sublimated.
Toxicological data
1. Acute toxicity
Rat caliber LD:>5 mg/kg; rat abdominal LD50: 2100 mg/kg;
Mouse caliber LD:>10 mg /kg; mouse intravenous LD50: 320 mg/kg;
2. Teratogenicity
Salmonella: 100ug/plate
3. Neurotoxicity
p>
Rabbit eye test: 500mg/24H
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 62.43
2. Molar volume (cm3/mol): 155.9
3. Isotonic specific volume (90.2K ): 465.2
4. Surface tension (dyne/cm): 79.2
5. Polarizability (10-24cm3): 24.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 8
6. Topological molecule polar surface area 74.6
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 342
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. What precipitates from acetic acid is orange crystal with a melting point of 200-203°C. Those that precipitate from diethyl ether are orange flake crystals. Those precipitated from ethanol, benzene, toluene and xylene are dark red needle crystals with a melting point of 196°C. Slightly soluble in water; soluble in concentrated sulfuric acid, sodium hydroxide solution, chlorobenzene, toluene, xylene, dichlorobenzene; soluble in appropriate amounts in ethanol, showing red color; soluble in ether, showing brown color and showing yellow fluorescence; soluble in caustic alkali solution and Ammonia is purple in color. It produces black precipitate when exposed to carbon dioxide, and 1g can be dissolved in about 13g of boiling glacial acetic acid. Can sublimate in high vacuum.
2.The toxicity of this product is similar to that of anthraquinone. The flame is corrosive when burning. Can cause allergies to skin. Operators should wear protective equipment. Rinse with plenty of water when in contact with skin. See anthraquinone.
Storage method
Keep sealed in a cool place. Packed in iron drums lined with plastic bags. Net weight per barrel is 20~50kg. Store in a dry and ventilated place. Should be protected from sun and moisture. Store and transport according to dangerous goods regulations.
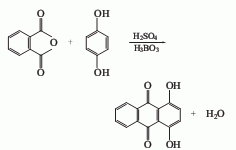
Synthesis method
Phthalic anhydride and hydroquinone are condensed in concentrated sulfuric acid in the presence of boric acid, and then diluted, washed, neutralized, oxidized and filtered to obtain the finished product. Raw material consumption quota: hydroquinone 550kg/t, phthalic anhydride 1280kg/t, boric acid 550kg/t, sulfuric acid 4300kg/t.

Purpose
This product is an important dye intermediate. It is dispersed brown GL (C.I.58050). Used to produce dispersed blue SR, dispersed blue B, dispersed dark blue RB, dispersed blue H3R, dispersed blue 5R, dispersed blue FFR, dispersed dye orange GL, transparent blue, reduced gray BG, reduced brown BR, etc.; can also be used to produce weak acid brilliants Blue RAW, weak acid green GS, weak acid bright yellow G, weak acid brilliant blue RAW, acid green P-3B, acid medium gray BS, acid anthraquinone blue R and intermediates used to produce intermediate 1,4-diaminoanthraquinone dye intermediates. 1,4-dihydroxyanthraquinone-2-sulfonic acid can be obtained by sulfonation of 1,4-dihydroxyquinone.