4-Methoxyphenol
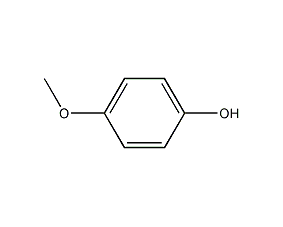
Structural formula
| Business number | 03YC |
|---|---|
| Molecular formula | C7H8O2 |
| Molecular weight | 124.14 |
| label |
p-hydroxyanisole, Hydroquinone monomethyl ether, Hydroquinone monomethyl ether, p-hydroxyanisole 4-hydroxyanisole, 1-Hydroxy-4-anisole, p-methoxyphenol, 4-Hydroxyanisole, Hydroquinone monomethyl ether, p-Hydroxyanisole, 1-Hydroxy-4-methoxy-benzene, p-Methoxyphenol, Polymerization inhibitor, UV inhibitors, antioxidants, Multifunctional solvent |
Numbering system
CAS number:150-76-5
MDL number:MFCD00002332
EINECS number:205-769-8
RTECS number:SL7700000
BRN number:507924
PubChem number:24878473
Physical property data
1. Character: white flake or waxy crystal
2. Relative density (g/mL, 20/4℃): 1.108872.3
3. Relative vapor density (g/mL, air=1): 4.3
4. Melting point (ºC): 55~57
5. Boiling point (ºC, normal pressure ): 243
6. Flash point (ºC): 132
7. Autoignition point or ignition temperature (ºC): 420
8. Saturated vapor Pressure (kPa, 20ºC): <0.0013
9. Solubility: Easily soluble in ethanol, ether, acetone, benzene and ethyl acetate, slightly soluble in water.
10. Relative density (25℃, 4℃): 0.958323.4
Toxicological data
1. Acute toxicity: Rat oral LD50: 1600 mg/kg; mouse intraperitoneal LD50: 250 mg/kg
2. Acute toxicity:
Oral LD50 1600mg /kg(rat)
Irritates the skin mild 6000mg(rbt)
Main irritant effect:
On the skin: Causes mild irritant effect
On eyes: Irritation effects
Sensitization: No known sensitizing effects
Ecological data
General remarks
Water hazard class 1 (German regulations) (self-assessment via list) The substance is slightly hazardous to water.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.
Do not exclude material without government permission�Surrounding environment.
Molecular structure data
1. Molar refractive index: 34.81
2. Molar volume (cm3/mol): 111.8
3. Isotonic specific volume (90.2K ): 278.9
4. Surface tension (dyne/cm): 38.6
5. Polarizability (10-24cm3): 13.80
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 75
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
2. Incompatible materials: bases, acid chlorides, acid anhydrides, and oxidants.
3. Exists in flue-cured tobacco leaves, oriental tobacco leaves and smoke.
Storage method
Store sealed in a dry and cool place.
Synthesis method
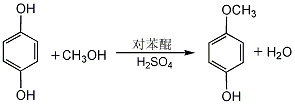
1. 4-Methoxyphenol can be produced by using hydroquinone as raw material, dimethyl sulfate as methylating agent, or methanol as methylating agent under high temperature, high pressure and catalysis. Another newer approach involves the reaction of hydroquinone with methanol in the presence of p-benzoquinone. Mix methanol and concentrated sulfuric acid evenly, add hydroquinone, heat to reflux, add p-benzoquinone methanol solution dropwise, and react for 3 hours. Cool, neutralize with 40% sodium hydroxide solution, and filter out the sodium sulfate precipitate. The filtrate recovers methanol and then extracts it with ether. The ether is evaporated from the extract, and then distilled under reduced pressure. The 110-112°C (0.53kPa) fraction is collected to obtain the finished product. The yield is 76%.

2.The p-nitroanisole method generates p-nitroanisole through a substitution reaction of p-nitrochlorobenzene; then it is reduced under the action of the reducing agent Na2S to generate p-aminoanisole; and finally it is regenerated Nitridation reaction decomposes into the product p-hydroxyanisole. The synthesis steps are as follows.
The synthesis of p-nitroanisole from p-nitrochlorobenzene will be equipped with a reflux condenser, stirrer and thermometer 250mL The three-necked flask was placed in a constant temperature bath, and 39.4g (0.25mol) p-nitrochlorobenzene and 100mLMethanol and 2g polyethylene glycol 800, heat and stir. When a large amount of methanol refluxes, add 68.2g 33.5% NaOH solution at a time, raise the temperature to (78±0.5)°C, and react 3h. Filter while hot into a beaker. After cooling slightly, add 60mL water into the beaker. Crystals will precipitate immediately. Collect by suction filtration, dry and weigh. 32.0g light yellow solid. The melting point is 54°C and the yield is 83.6%.
Synthesize p-nitroanisole to prepare 60g of 22%~23% Na2S solution. After heating to 95℃, add 10g of p-nitroanisole Anisole, stir, carefully heat to 100°C, emulsify and reduce p-nitroanisole in Na2S solution, react for 6 hours. Then the solution settles, the lower alkali liquid is discarded, the crude para-aminoanisole is washed with hot water 3 to 4 times, left to stand, filtered while hot, cooled slightly, crystallized and filtered, dried, and transferred to 50 mL Distill under reduced pressure in a Kirschner flask, and collect the 145-150°C fraction at 5333-6665Pa to obtain 6.5g of a reddish solid. The melting point is 56~57℃, and the yield is 81%.
3. Tobacco: OR, 26; FC, 18 .
Purpose
Used as polymerization inhibitor, UV inhibitor, dye intermediate for vinyl plastic monomers, and antioxidant BHA (3-tert-butyl-4-hydroxyanisole) used in the synthesis of edible oils and cosmetics, etc. .