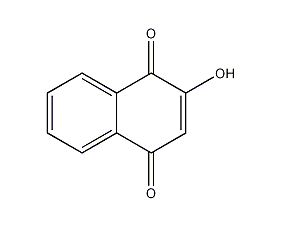
5,8-dihydroxy-1,4-naphthoquinone
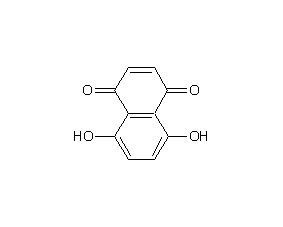

Structural formula
| Business number | 0519 |
|---|---|
| Molecular formula | C10H6O4 |
| Molecular weight | 190.16 |
| label |
Naxi, 1,4-dihydroxynaphthoquinone, 5,8-Dihydroxy-1,4-naphthalenedione, Naphthazarin |
Numbering system
CAS number:475-38-7
MDL number:MFCD00001685
EINECS number:207-495-4
RTECS number:QL7970000
BRN number:880561
PubChem number:24851613
Physical property data
1. Character:Undetermined
2. Density (g/ m3,25/4℃): Undetermined
3. Relative vapor density (g/cm3,AIR=1): Undetermined
4. Melting point (ºC):220-230
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive index : Undetermined
8. Flash Point (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined
12. Saturated vapor pressure (kPa,60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Water) logarithmic value of the partition coefficient: undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility: Undetermined
Toxicological data
Mutagenicity data: microbial organismsTESTSystemic mutation: bacteria–Salmonella Typhimurium:100ug/Tablet
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1、 Molar refractive index:46.66
2、 Molar Volume(m3/mol):119.4
3、 Isotonic specific volume (90.2K): 360.9
4、 Surface tension(dyne/cm):83.5
5、 Polarizability(10-24cm3):18.50
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.8
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 6
6. Topological molecule polar surface area 74.6
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 277
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Use and store according to specifications, will not decompose, and avoid contact with oxides
Storage method
Save in a sealed manner and place it in a ventilated and dry place to avoid contact with other oxides.
Synthesis method
None
Purpose
None