1,4-Naphthoquinone 1,4-Naphthoquinone


Structural formula
| Business number | 03MD |
|---|---|
| Molecular formula | C10H6O2 |
| Molecular weight | 158 |
| label |
1,4-naphthalenedione, α-Naphthoquinone, 1,4-Naphthoquinone, 1,2-naphthalenedione, Beta-naphthoquinone, 1,4-Naphthoquinone, 1,4-naphthoquinone, p-Naphthoquinone, α-Naphthoquinone |
Numbering system
CAS number:130-15-4
MDL number:MFCD00001676
EINECS number:204-977-6
RTECS number:QL7175000
BRN number:878524
PubChem number:24858619
Physical property data
1. Properties: bright yellow needle-like crystals. It can evaporate with water vapor and has the smell of benzoquinone.
2. Density (g/mL, 25/4℃): 1.422
3. Melting point (℃): 128.5
4. Solubility: easy Soluble in hot ethanol, ether, benzene, chloroform, carbon disulfide and acetic acid, soluble in alkaline hydroxide solution, slightly soluble in petroleum ether, and a small amount soluble in cold water.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 42.90
2. Molar volume (cm3/mol): 122.5
3. Isotonic specific volume (90.2K ): 330.5
4. Surface tension (dyne/cm): 52.9
5. Polarizability (10-24cm3): 17.00
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 227
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Toxic and irritating.
2. It can be sublimated by heating to 100℃. Strong heating can decompose and explode.
Storage method
None yet
Synthesis method
Using 1-naphthol, 1-naphthylamine, 1,4-aminonaphthol, naphthalene, etc. as raw materials, naphthoquinone can be obtained through oxidation. The oxidizing agent can be chromic anhydride, sodium dichromate, potassium dichromate, air, etc. An industrially more advantageous method is to produce naphthoquinone by catalytic oxidation of naphthalene in the presence of barium pentoxide.
Purpose
1. Used for anthraquinoneProperty. 2,3-dichloronaphthoquinone produced by the chlorination reaction of naphthoquinone is an important agricultural fungicide, used to control wheat smut, rice blast, potato late blight and damping off of vegetable seedlings.
2. Mainly used as dienophile to prepare various aromatic ring compounds through Diels-Alder cycloaddition reaction. It can also realize amination reaction, allylation reaction, cyclooligomerization reaction and Thiele-Winter acetation reaction produces other types of aromatic compounds.
Due to the electron-withdrawing effect of the two carbonyl groups at the 1-position and 4-position, 1,4-naphthoquinone is a very effective dienophile and can react with electronegative diene compounds. -Alder cycloaddition reaction to obtain benzo polycyclic compounds. Such as the cycloaddition reaction with benzofuran to prepare tetracenequinone (formula 1)[1], or the reaction with 2,3-vinyl indole to prepare pentacene (formula 2)[2].

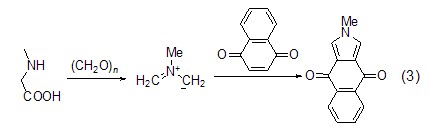
1,4-naphthoquinone also A new method is provided for the synthesis of 2H-isoindole-4,7-dione (formula 3)[3].
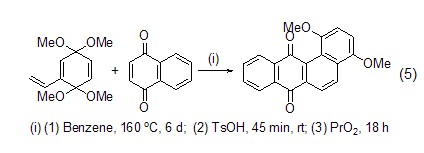
In the synthesis of biologically active compounds On the other hand, 1,4-naphthoquinone also plays a very important role. For example, the reaction with pyrrole provides a good precursor for the preparation of antibiotics (Formula 4)[4], and the Diels-Alder reaction with vinylquinone acetal is used to synthesize non-linear benzopolycyclic rings. Antibiotics provide a good method (Formula 5)[5].

Allyl cyanide can also undergo cycloaddition reaction with 1,4-naphthoquinone (formula 6)[6]. 3-Alkenylpyrrole as a diene can also react with 1,4-naphthoquinone to obtain indole compounds (Formula 7)[7].
