4,4′-Oxydibenzenesulfonyl hydrazide 4,4′-Oxydibenzenesulfonyl hydrazide
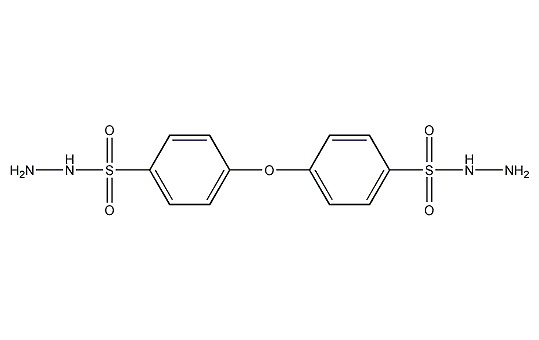
Structural formula
| Business number | 01R6 |
|---|---|
| Molecular formula | C12H14N4O5S2 |
| Molecular weight | 358.39 |
| label |
4,4′-p-sulfonyl hydrazide diphenyl ether, 4,4′-Oxybis(benzenesulfonyl hydrazide),, Cross-linking agent |
Numbering system
CAS number:80-51-3
MDL number:None
EINECS number:201-286-1
RTECS number:DB7321000
BRN number:None
PubChem ID:None
Physical property data
1. Properties: White needle-like crystals, flammable. Irritating
2. Density (g/mL, 25/4℃): 1.52
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 160.0℃~161.0℃
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, normal pressure) 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation ( º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain Determine
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17 . Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Slightly soluble in heat Water and ethanol, insoluble in cold water and various organic solvents
Toxicological data
1. Teratogenicity
Salmonella: 800ug/plate; rat liver: 100 umol/L
Mouse liver: 100 umol/L
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 85.38
2. Molar volume (cm3/mol): 235.5
3. Isotonic specific volume (90.2K ): 669.5
4. Surface tension (dyne/cm): 65.2
5. Polarizability (10-24cm3): 33.84
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.1
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 9
p>
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 170
7. Number of heavy atoms: 23
8. Surface charge: 0
9. Complexity: 516
10. Number of isotope atoms: 0
11. Determine the atomic position Number of stereocenters: 0
12. Uncertain number of stereocenters of atoms: 0
13. Determined number of stereocenters of chemical bonds: 0
14. No Determine the number of stereocenters of chemical bonds: 0
15. The number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed and stored away from light.
Synthesis method
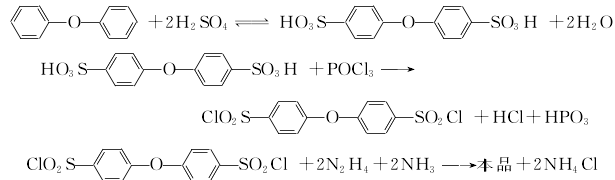
1.Use diphenyl ether to undergo sulfonyl chlorination with chlorosulfonic acid to obtain 4,4′-oxobisphenylsulfonyl chloride, and then react with hydrazine hydrate to obtain 4,4′-Oxobishenylsulfonylhydrazide. The reaction formula is as below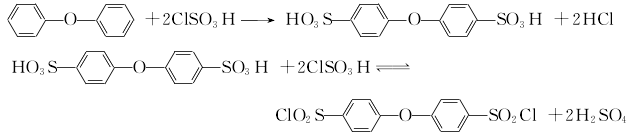
Use diphenyl ether and excess chlorosulfonic acid (molar ratio about 1:6) to react at room temperature for 2 hours Then pour it into water and filter to obtain the crude intermediate 4,4′-chlorobisphenylsulfonyl chloride. Diphenyl ether was sulfonated with approximately the same amount of concentrated sulfuric acid under vacuum and 135°C for 4 hours, then refluxed with an equal amount of phosphorus oxychloride for 3 hours, poured into ice water, separated, filtered, washed, filtered and dried. Get intermediate. The intermediate is reacted with a slight excess of hydrazine hydrate and an equivalent amount of ammonia water at 30 to 40°C for 3 hours, and finally the product is filtered, washed, and dried to obtain the product.
2.Diphenyl ether is first sulfonated with concentrated sulfuric acid, and then reacted with phosphorus oxychloride to obtain 4,4′- Oxybiphenylsulfonyl chloride continues to react with hydrazine hydrate and ammonia to obtain 4,4′-oxobisphenylsulfonyl hydrazide. The reaction formula is as follows

Add the predetermined amount of diphenyl ether and concentrated sulfuric acid into the reaction bottle, connect the pressure reduction system and start to slowly raise the temperature to 135°C. After maintaining the reaction for 4 hours, the temperature was lowered to 100-110°C and the pressure reduction system was removed. Add the predetermined amount of phosphorus oxychloride dropwise from the liquid funnel, maintain the reaction for about 3 hours and then cool down. Precipitate the reactant in ice water. After washing the precipitate, take a certain amount of the precipitate and place it in another reaction bottle. Add a certain amount of water and start stirring. Add a mixture of ammonia water and hydrazine hydrate, maintain the reaction at 40°C, filter the reactant after about 3 hours, wash it with water until neutral, and dry it under vacuum or infrared to obtain an off-white powder product.
Purpose
Foaming agent for rubber and plastic industry.
It is an organic sulfonyl hydrazide foaming agent that is widely used and is known as a universal foaming agent. It can be used in polyvinyl chloride, polyethylene, polypropylene and ABS resin, etc. It can also be used as a blend of rubber and synthetic resin and as a foaming agent for various synthetic rubbers. Applicable products include wallpaper, carpets, communication cables, artificial leather and various packaging materials. Its cell structure is fine and uniform, and it is especially suitable for manufacturing various foam plastics such as polyethylene foamed wire and cable insulation materials and microporous PVC paste foam. Used together with sodium bicarbonate to reduce the decomposition temperature. When used in chloroprene rubber, it also has the effect of promoting vulcanization.