2,5-Dimethoxyaniline 2,5-Dimethoxyaniline
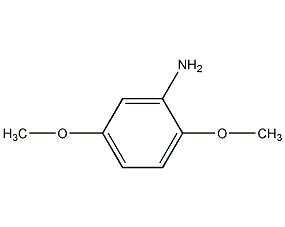
Structural formula
| Business number | 02M9 |
|---|---|
| Molecular formula | C8H11NO2 |
| Molecular weight | 153 |
| label |
Aminohydroquinone dimethyl ether, 2-amino-1,4-dimethoxybenzene, o-Aminoterephthalene, Aminohydroquinone dimethyl ether, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:102-56-7
MDL number:MFCD00008368
EINECS number:203-040-9
RTECS number:BX4375000
BRN number:776823
PubChem number:24847125
Physical property data
1. Properties: gray flaky solid.
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 69~73
5. Boiling point (ºC, partially decomposed): 270
6. Boiling point (ºC, 7mmHg): 140
7. Refractive index: Undetermined
8. Flash point (ºC): 130
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 40ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V) : Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in cold water, soluble in organic solvents and hot water.
Toxicological data
Acute toxicity: Oral LD50 in mice: 120mg/kg; Oral LD50 in wild birds: 100mg/kg.
Main irritant effects:
On skin: May cause inflammation.
On eyes: May cause irritation.
Sensitization: No known sensitizing effects.
Toxic, irritating skin and mucous membranes, and can cause allergies.
Ecological data
General Notes
Do not allow this product to come into contact with groundwater, waterways or sewage systems.
Water hazard class 2 (German Regulation) (self-assessment via list) The substance is hazardous to water.
Even small amounts of product seeping into the ground can pose a hazard to drinking water
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 43.84
2. Molar volume��cm3/mol): 139.7
3. Isotonic specific volume (90.2K): 346.4
4. Surface tension (dyne/cm): 37.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 17.38
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 44.5
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 119
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxidizing agents. The color turns darker when exposed to light. Alkaline. This product is highly toxic, irritates skin and mucous membranes, and can cause allergies.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire, heat sources and anti-static. Protect from direct sunlight. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
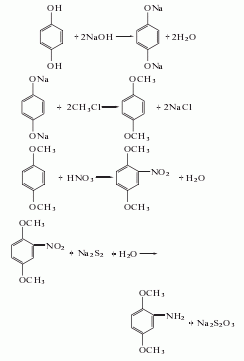
Hydroquinone is dissolved in liquid alkali to form hydroquinone sodium salt. Hydroquinone sodium salt and methyl chloride react at 75-120℃ and 1.3-1.7MPa pressure to generate p-dimethoxybenzene, which is then nitrated with nitric acid to generate 2,5-dimethoxynitrobenzene. It is reduced with sodium polysulfide and finally obtained by cooling, filtering and washing with water.

Purpose
Mainly used as an intermediate for the preparation of ice dye black salt K. It is also used as an intermediate for medicines and pesticides, and for the preparation of antioxidants, etc.