1,3,5-Trinitrobenzene 1,3,5-Trinitrobenzene
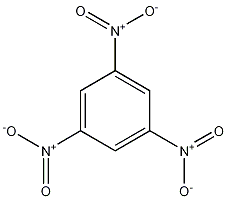

Structural formula
| Business number | 02FP |
|---|---|
| Molecular formula | C6H3N3O6 |
| Molecular weight | 213.1 |
| label |
Symmetric trinitrobenzene, s-Trinitrobenzene, Tnb, 1,3,5-Trinitro-benzen, Benzene,1,3,5-Trinitro-, benzite, Rcra waste number u234, Rcrawastenumberu234, S-trinitrobenzene |
Numbering system
CAS number:99-35-4
MDL number:MFCD00059179
EINECS number:202-752-7
RTECS number:DC3850000
BRN number:None
PubChem ID:None
Physical property data
1.Characteristics: white or yellow orthorhombic crystal. [1]
2. Melting point (℃): 123[2]
3. Boiling point (℃): 315 (Decomposition) [3]
4. Relative density (water = 1): 1.76[4]
5. Saturated vapor pressure (kPa): 0.27 (175℃)[5]
6. Heat of combustion (kJ/mol): -2774.3[6]
7. Critical temperature (℃): 562[7]
8. Critical pressure (MPa): 3.39[8]
9. Octanol/water partition coefficient: 1.10[9]
10. Solubility: insoluble in water, slightly soluble in heat Ethanol is easily soluble in ether, acetone and benzene. [10]
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit eye contact, 100mgREACTION SEVERITY, strong reaction;
2. Acute toxicity: rat oral LD50: 275mg/kg; small Mouse oral LD50: 572mg/kg; intravenous injection LD50 in mice: 32mg/kg; rabbit skin contact LD: >2mg/kg; guinea pig oral LD50: 730mg/kg;
3. Other multiple doses Toxicity: Rat oral TDLo: 3640μg/kg/26W-I; Rat oral TDLo: 710mg/kg/10D-I; Rat oral TDLo: 2070mg/kg/90D-C; Rat oral TDLo: 355mg/kg/10D-I; Oral TDLo for dogs: 400mg/kg/4D-I;
4. Mutagenicity: Mutant microorganism test: bacteria – Salmonella typhimurium, 10μg/plate;
p>
5. Acute toxicity [11] LD50: 275mg/kg (oral in rats); 572mg/kg (oral in mice); 730mg/ kg (guinea pig oral)
6. Irritation [12] Rabbit eye: 100mg, severe irritation.
7. Mutagenicity [13] Microbial mutagenicity: Salmonella typhimurium 10μg/dish.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[14] This substance is harmful to the environment and should be treated with special Pay attention to water pollution.
Molecular structure data
1. Molar refractive index: 45.88
2. Molar volume (cm3/mol): 124.9
3. Isotonic specific volume (90.2K ): 373.7
4. Surface tension (dyne/cm): 80.0
5. Polarizability: 18.19
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 138
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 229
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[15] Stable
2. Incompatible substances[16] Strong oxidants, ammonia, amines, etc.
3. Conditions to avoid contact[17] Heat, impact, friction, vibration
4. Polymerization hazard[18] No polymerization
5. Decomposition products[19] Nitrogen oxides
Storage method
Storage Precautions[20] Store in a cool, dry and ventilated warehouse dedicated to explosives. The storage temperature does not exceed 32°C and the relative humidity does not exceed 80%. If it contains water as a stabilizer, the storage temperature should not be lower than 1°C, the relative humidity should be less than 80%, and it should be kept away from fire and heat sources. They should be stored separately from oxidants, reducing agents, active metal powders and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. Suitable materials should be available in the storage area to contain spills. Vibration, impact and friction are prohibited.
Synthesis method
None yet
Purpose
Used as an explosive and as a pH indicator in analytical chemistry. [21]