8-amino-2-naphthalenesulfonic acid
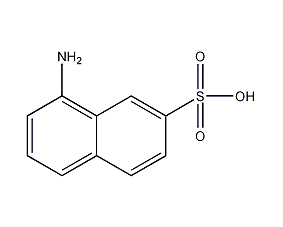

Structural formula
| Business number | 03AN |
|---|---|
| Molecular formula | C10H9NO3S |
| Molecular weight | 223.25 |
| label |
1-Naphthylamine-7-sulfonic acid, Cleves acid-1,7, H2NC10H6SO3H, aromatic compounds |
Numbering system
CAS number:119-28-8
MDL number:MFCD00004032
EINECS number:204-311-4
RTECS number:None
BRN number:2115629
PubChem number:24891278
Physical property data
1. Properties: light gray crystal.
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): ≥300
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 12mmHg): Undetermined
p>
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,ºC): Undetermined
12. Saturated vapor pressure ( kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Hardly soluble in water, soluble in water at 25°C 220 parts of water, very slightly soluble in ethanol and ether, soluble in alkali solution. It appears blue in ferric chloride solution.
Toxicological data
Toxic, rat oral LD50: 14mg/kg.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 58.27
2. Molar volume (cm3/mol): 148.6
3. Isotonic specific volume (90.2K): 433.1
4. Surface tension (dyne/cm): 72.1
5. Polarizability (10-24cm3): 23.10
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP):1.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
p>
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 80.4
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 322
10. Number of isotope atoms: 0
11. Determine atomic configuration Number of centers: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain Number of stereocenters of chemical bonds: 0
15, Number of covalent bond units: 1
Properties and stability
The raw material naphthalene is toxic, and corrosive raw materials such as concentrated sulfuric acid and concentrated nitric acid are used in production. The equipment should be sealed to prevent leakage. Operators should wear labor protection equipment and maintain good ventilation in the workshop.
Storage method
Synthesis method
After sulfonation of refined naphthalene, it is nitrated with mixed acid to obtain a 1-nitronaphthalene sulfonic acid mixture, which is neutralized with magnesium carbonate to obtain a mixture of three isomers: 1-nitronaphthalene-6-magnesium sulfonate, 1-nitronaphthalene-6-magnesium sulfonate Magnesium naphthalene-7-sulfonate and magnesium 1-nitro-8-sulfonate. The three isomer mixtures are reduced with iron powder to obtain the corresponding three isomers of magnesium aminonaphthalene sulfonate. These three magnesium salts are then separated based on their different solubilities at different temperatures and acidities. After further acidification, three products are obtained simultaneously. The refined naphthalene is put into the sulfonation kettle for sulfonation, neutralized and reduced to obtain 1-naphthylamine-6-magnesium sulfonate, 1-naphthylamine-7-magnesium sulfonate and 1-naphthylamine-8-magnesium sulfonate. After the reduction reaction is completed, filter and wash the filter residue with hot water. The washing liquid and filtrate are sent to the concentration kettle, concentrated to 26.5~27.2g/L, slowly cooled to 30~32°C within 3 hours, 1-naphthylamine-6-magnesium sulfonate is precipitated, and filtered. The filter cake is subjected to acid precipitation to obtain 1-naphthylamine-6-sulfonic acid. The filtrate was heated to 40~45°C, acidified by adding sulfuric acid, and stirred for 12 hours to precipitate 1,8-naphthylamine sulfonic acid. Filter and wash with 60°C hot water. Combine the washing liquid and filtrate, heat to 85°C, add 50% to 60% sulfuric acid, acidify until the Congo red test paper shows obvious blue color, cool to 60°C, and keep warm for 0.5h to precipitate 1-naphthylamine-7-sulfonic acid. Filter and wash with hot water at 60~70°C to obtain 1-naphthylamine-7-sulfonic acid wet material.
Purpose
This product is a dye intermediate and is used to manufacture direct light fast blue B2RL, direct light fast red brown RTL, sulfide blue CV and other dyes.