Acriflavine
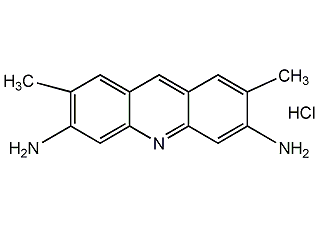

Structural formula
| Business number | 03Q5 |
|---|---|
| Molecular formula | C15H16ClN3 |
| Molecular weight | 273.76 |
| label |
2,7-Dimethyl-3,6-acridinediamine monohydrochloride, 3,6-Diamino-2,7-dimethylacridine hydrochloride, Acriflavine G, Azanthracene yellow, Green light alkaline yellow, 2,6-Diamino-2.7-dimethylacridine hydrochloride, Acridine Yellow G, biological stains |
Numbering system
CAS number:135-49-9
MDL number:MFCD00012661
EINECS number:205-194-2
RTECS number:AR8790000
BRN number:None
PubChem number:24851921
Physical property data
1. Appearance: yellow crystalline powder .
2. Density ( g/mL,25/4℃) : Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point ( ºC): 400
5. Boiling point ( ºC,Normal pressure): Undetermined
6. Boiling point ( ºC, 5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º ): Undetermined
10. Autoignition point or ignition temperature (ºC): Not OK
11. Vapor pressure (kPa,25ºC): Undetermined
12. Saturation vapor pressure (kPa,60ºC): Undetermined
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 10
6. Topological molecular polar surface area (TPSA):64.9
7. Number of heavy atoms: 19
8. Surface charge: -1
9. Complexity: 278
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Yellow crystalline powder. When dissolved in hot water and ethanol, it turns orange with green fluorescence. When exposed to concentrated sulfuric acid, it turns light yellow. After dilution, a yellow precipitate is produced. When sodium hydroxide is added to the aqueous solution, a yellow precipitate is produced. When nitrous acid is added to the aqueous solution, it turns dark purple. The pH of a 1% aqueous solution is 5.4. The maximum absorption wavelength is 442nm. The median lethal dose (mouse, subcutaneous) is 280 mg/kg.
Storage method
Keep sealed and dry.
Synthesis method
None
Purpose
Biological stain. Used for fluorescent staining in insect histology. Prepare culture medium.
�体”>11. Determine the number of atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Yellow crystalline powder. When dissolved in hot water and ethanol, it turns orange with green fluorescence. When exposed to concentrated sulfuric acid, it turns light yellow. After dilution, a yellow precipitate is produced. When sodium hydroxide is added to the aqueous solution, a yellow precipitate is produced. When nitrous acid is added to the aqueous solution, it turns dark purple. The pH of a 1% aqueous solution is 5.4. The maximum absorption wavelength is 442nm. The median lethal dose (mouse, subcutaneous) is 280 mg/kg.
Storage method
Keep sealed and dry.
Synthesis method
None
Purpose
Biological stain. Used for fluorescent staining in insect histology. Prepare culture medium.