Allyl methacrylate Allyl methacrylate
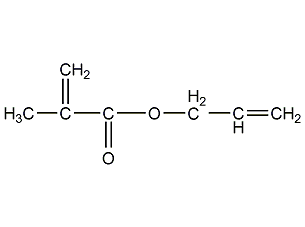

Structural formula
| Business number | 02AB |
|---|---|
| Molecular formula | C7H10O2 |
| Molecular weight | 126.15 |
| label |
Allyl methacrylate, Allyl methacrylate, Thacrylic acid allyl ester |
Numbering system
CAS number:96-05-9
MDL number:MFCD00008592
EINECS number:202-473-0
RTECS number:UD3483000
BRN number:1747406
PubChem number:24882664
Physical property data
1. Properties: slightly yellow liquid.
2. Density (g/mL, 20℃): 0.938
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): -65
5. Boiling point (ºC, normal pressure): 144
6. Boiling point (ºC, 43mmHg): 59-61
7. Refractive index (n20): 1.436
8. Flash point (ºC): 37
9. Specific rotation ( º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 20ºC): 4.6
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Easily soluble in most organic matter Solvent, almost insoluble in water.
Toxicological data
1. Skin/eye irritation
Standard Draize test: rabbit, skin contact: 20mg/24H, severity of reaction: moderate.
Standard Draize test: Rabbit, eye contact: 500mg/24H, severity of reaction: mild.
2. Acute toxicity: Rat oral LD50: 70mg/kg; Rat inhalation LC50: 1800mg/m3; Mouse oral LD50: 57mg/kg; Mouse inhalation LC50: 5500mg/m3; rabbit skin contact LD50: 500μg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 35.54
2. Molar volume (cm3/mol): 137.5
3. Isotonic specific volume (90.2K ): 309.5
4. Surface tension (dyne/cm): 25.6
5. Polarizability (10-24cm3): 14.09
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.7
2. Number of hydrogen bond donors���0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 26.3
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 136
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. No Determine the number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants.
2. Flammable, harmful when taken orally or in contact with skin, and irritating to eyes, respiratory system and skin.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire, heat sources and anti-static. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
It can be obtained from the reaction of acrylamide and allyl alcohol under the action of concentrated acid and H2O2, or it can be obtained from allyl acetate and methyl methacrylate. Derived from transesterification. It can also be produced by reacting methacrylic acid with allyl alcohol, using p-toluenesulfonic acid as a catalyst, and refluxing in the presence of the polymerization inhibitor hydroquinone. The generated water is continuously removed during the reaction. After the reaction is completed, the excess is recovered by fractionation. Allyl alcohol, the product was purified by distillation under reduced pressure.
Purpose
1. Widely used as comonomer, grafting monomer and cross-linking agent for tooth repair in the preparation of organic glass.