Benzyl cinnamate Benzyl cinnamate
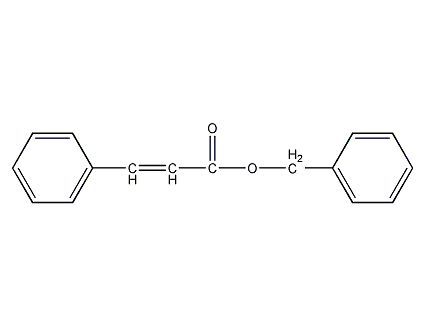

Structural formula
| Business number | 02NH |
|---|---|
| Molecular formula | C16H14O2 |
| Molecular weight | 238.28 |
| label |
Benzyl Phenylacrylate, cinnamic acid benzylic acid, Cinnamic acid benzyl ester, 3-Phenyl-2-propenoic acid phenylmethyl ester, trans-Cinnamic acid benzyl ester, UV absorber |
Numbering system
CAS number:103-41-3
MDL number:MFCD00004789
EINECS number:203-109-3
RTECS number:GD8400000
BRN number:2051339
PubChem number:24890293
Physical property data
1. Properties: white crystal. Has a weak, sweet balsamic aroma.
2. Density (g/mL, 20/4℃): 1.128
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 37~39
5. Boiling point (ºC, normal pressure): 195~200 (666.5pa)
6. Boiling point (ºC, normal pressure) 2.93kPa): 228~230
7. Refractive index (n20D): 1.4035
8. Flash point (ºC): >100
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper explosion limit (%, V/V): Undetermined
18. The lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol, ether and oils, almost insoluble in water, propylene glycol and glycerin.
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit skin contact, 500mg/24H reaction severity, mild reaction; 2. Acute toxicity: rat oral LD50: 5530mg/kg; guinea pig oral LD50: 3760mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 73.03
2. Molar volume (cm3/mol): 211.0
3. Isotonic specific volume (90.2K ): 546.8
4. Surface tension (dyne/cm): 45.0
5. Dielectric constant:
6. Dipole moment (10– 24cm3):
7. Polarizability: 28.95
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 3.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 5
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 26.3
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 271
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants.
2. Exist in smoke.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. 2. Equip with corresponding varieties and quantities of fire-fighting equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Mainly adopt compounding method and condensation method. The esterification method uses cinnamic acid as raw material, first neutralizes it with sodium hydroxide, and then performs esterification reaction with chlorotoluene. The condensation method is obtained by condensation reaction using benzyl acetate and benzaldehyde as raw materials.
2.Using natural product extraction method and cinnamic acid synthesis method. The natural product extraction method is obtained by extracting benzyl cinnamate that exists in balsam of Peru, turlu balsam and benzoin. The cinnamic acid synthesis method uses cinnamic acid as raw material, first neutralizes it with sodium hydroxide solution, then performs esterification reaction with chlorotoluene, and finally refines it.
3. Direct esterification method of cinnamic acid and benzyl alcohol: method of transesterification using benzyl alcohol and methyl cinnamate: method of interaction between sodium cinnamate and benzyl chloride: by acetic acid Claisen condensation of benzyl esters and benzoic acid.
Purpose
1. Ester synthetic fragrances. It is mainly used as a fixative for oriental essences and artificial cologne, and can also be used as a soap fragrance.
2.UV absorber. Mainly used in sunscreen and tanning cosmetics. The maximum absorption wavelength of ultraviolet light is 310nm. The amount added is usually less than 5%. In addition, it is also used to prepare artificial ambergris and oriental flavors as fixatives. It is also used as a raw material for flavoring soaps and cosmetics.
3. Used in food flavor formulations, often used to prepare apricot, cherry, pineapple and other fruity flavors.