BPB
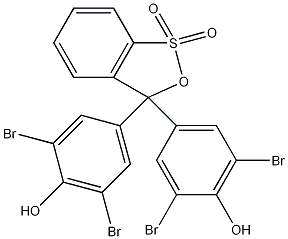
Structural formula
| Business number | 037Y |
|---|---|
| Molecular formula | C19H10Br4O5S |
| Molecular weight | 669.96 |
| label |
Tetrabromophenol sulfonphthalein, Tetrabromophenol sulfonyl phthalide, Tetrabromophenolsulfonphenolphthalein, 3′,3”,5′,5”-Tetrabromophenolsulfonephthalein, indicator |
Numbering system
CAS number:115-39-9
MDL number:MFCD00005875
EINECS number:204-086-2
RTECS number:SJ7453000
BRN number:61698
PubChem number:24891504
Physical property data
1. Properties: light yellow to brown powder.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 279℃ (decomposition).
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition Combustion temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in sodium hydroxide solution, soluble in methanol, ethanol and benzene, slightly soluble in water (approx. 0.4g/100ml). Its sodium salt is soluble in water.
Toxicological data
None
Ecological data
No harm to water bodies.
Molecular structure data
1. Molar refractive index: 123.26
2. Molar volume (cm3/mol): 304.5
3. Isotonic specific volume (90.2K): 893.7
4. Surface tension (dyne/cm): 74.1
5. Polarizability (10-24cm3): 48.86
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 5
p>
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecule polar surface area 92.2
7. Number of heavy atoms: 29
8. Surface charge: 0
9. Complexity: 662
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxides.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources.
2. It should be stored separately from oxidants, etc., and avoid mixed storage.
3. Equipped with corresponding varieties and quantities of fire-fighting equipment.
4. The storage area should be equipped with leakage emergency treatment equipment and suitable containment materials.
Synthesis method
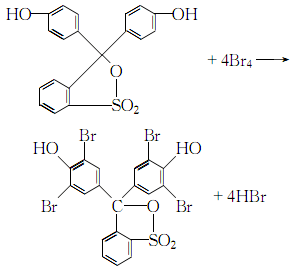
 1. Dissolve phenol red in glacial acetic acid, add bromine and dissolve in glacial acetic acid while stirring The solution was stirred for a few minutes, poured into 60°C hot water, cooled to room temperature, and left overnight. Filter, wash the filter cake with glacial acetic acid and benzene in sequence, and dry it to obtain bromophenol blue. 2.Dissolve phenol red in glacial acetic acid, heat to boiling, add dropwise a solution of bromine dissolved in glacial acetic acid, when a yellow solid precipitates, filter and wash it away with acetic acid Free bromine can be dried in the air to obtain the crude product. Recrystallize with glacial acetic acid or a mixed solvent of acetone and glacial acetic acid to obtain pure bromophenol blue. 3.Dissolve phenol red and bromine in acetic acid to make a solution. First heat the phenol red acetic acid solution to boiling, then slowly add the bromine acetic acid solution while stirring:When the phenol red reaction is complete and yellow crystals no longer precipitate , cooling, filtering. After washing away the free bromine in the crystal with a small amount of acetic acid, recrystallize it with acetic acid or acetone and dry it in the air to obtain pure bromophenol blue.
1. Dissolve phenol red in glacial acetic acid, add bromine and dissolve in glacial acetic acid while stirring The solution was stirred for a few minutes, poured into 60°C hot water, cooled to room temperature, and left overnight. Filter, wash the filter cake with glacial acetic acid and benzene in sequence, and dry it to obtain bromophenol blue. 2.Dissolve phenol red in glacial acetic acid, heat to boiling, add dropwise a solution of bromine dissolved in glacial acetic acid, when a yellow solid precipitates, filter and wash it away with acetic acid Free bromine can be dried in the air to obtain the crude product. Recrystallize with glacial acetic acid or a mixed solvent of acetone and glacial acetic acid to obtain pure bromophenol blue. 3.Dissolve phenol red and bromine in acetic acid to make a solution. First heat the phenol red acetic acid solution to boiling, then slowly add the bromine acetic acid solution while stirring:When the phenol red reaction is complete and yellow crystals no longer precipitate , cooling, filtering. After washing away the free bromine in the crystal with a small amount of acetic acid, recrystallize it with acetic acid or acetone and dry it in the air to obtain pure bromophenol blue.
Purpose
1. Used as an acid-base indicator, with a pH discoloration range of 3.0 (yellow) to 4.6 (purple). It can also be used as an adsorption indicator.
2.It is also used as a reagent for the determination of aliphatic carboxylic acids, sugar alcohols and chlorine-containing pesticides by thin layer chromatography, and for the photometric determination of quaternary ammonium cations. Extraction separation agent.