bromocresol purple
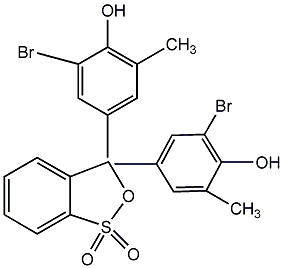
Structural formula
| Business number | 037Z |
|---|---|
| Molecular formula | C21H16Br2O5S |
| Molecular weight | 540.22 |
| label |
Dibromo-o-cresolsulfonphthalein, Dibromo-o-cresolsulfonyl phthalide, Dibromo-o-cresolsulfonphthalide, 5,5′-Dibromo-o-cresolsulfonphthalein, Bromcresol purple sultone form, indicator |
Numbering system
CAS number:115-40-2
MDL number:MFCD00011681
EINECS number:204-087-8
RTECS number:None
BRN number:359618
PubChem number:24847168
Physical property data
1. Properties: light yellow fine crystals.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 241~242
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol and dilute alkali solution. Almost insoluble in water.
Toxicological data
None
Ecological data
No harm to water bodies.
Molecular structure data
1. Molar refractive index: 117.07
2. Molar volume (cm3/mol): 304.7
3. Isotonic specific volume (90.2K ): 868.0
4. Surface tension (dyne/cm): 65.8
5. Polarizability (10-24cm3): 46.41
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 5
4. RotatableNumber of chemical bonds: 2
5. Number of tautomers: 5
6. Topological molecule polar surface area 92.2
7. Number of heavy atoms: 29
8. Surface charge: 0
9. Complexity: 680
10. Number of isotope atoms: 0
11. Determine Number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14 .The number of uncertain chemical bond stereocenters: 0
15. The number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxides.
2.Soluble in ethanol and dilute alkali solution, the ethanol solution is green. Reacts with dibasic hydroxy acid to produce yellow color. It forms an ionic association with quaternary ammonium cations and can be extracted with organic solvents.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, etc. and avoid mixed storage.
2. Equip with corresponding varieties and quantities of fire-fighting equipment.
3. The storage area should be equipped with leakage emergency treatment equipment and suitable containment materials.
Synthesis method
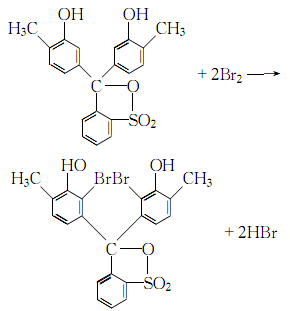
1. Dissolve o-toluidine in dilute sulfuric acid, cool, add sodium nitrite solution while stirring, and control the reaction temperature below 5°C. After the reaction is completed, heat to 40-50°C until no bubbles occur, cool and filter, and fractionate and refine o-cresol. Mix o-cresol with o-toluenesulfonic anhydride and zinc chloroform, stir and heat, react at 115-120°C for 7 hours, remove the remaining o-cresol with steam, and filter to obtain o-cresol red: o-cresol red is refined and dissolved in For glacial acetic acid, add the mixed solution of bromine and glacial acetic acid under stirring. After the addition is completed, heat at 40-50°C for 4 hours, cool and filter to obtain bromocresol purple.
2.Dissolve the reagents cresyl red and bromine in acetic acid respectively to make a solution, then add the bromine acetic acid solution under stirring. Cresol red in acetic acid solution:

Stir continuously for more than 4 hours at 40~50℃, stop heating, filter after cooling, wash the crystal with benzene and drain it, after drying it is methyl bromide Phenol purple.
Purpose
1. Acid-base indicator, the color changes from light yellow to purple, and the pH value of the color change range is 5.2-6.8.Used for thin layer chromatography to detect dihydroxy acids and halogen ions (except fluorine). It is also used as an extraction agent for the photometric determination of quaternary ammonium cations.