Butyl butyrate Butyl butyrate
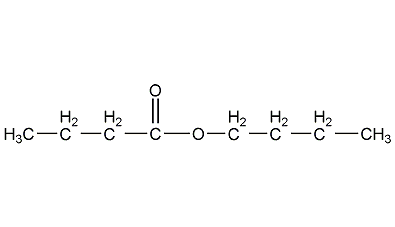
Structural formula
| Business number | 02YV |
|---|---|
| Molecular formula | C8H16O2 |
| Molecular weight | 144.21 |
| label |
n-butyl butyrate, n-butyl n-butyrate, n-Butylbutyrate, n-butyl butyrate, butyl butyrate, 1-Butyl butyrate, Butanoic acid butyl ester, Butyl n-butyrate, CH3CH2CH2COOC4H9, paint solvents, spices |
Numbering system
CAS number:109-21-7
MDL number:MFCD00009450
EINECS number:203-656-8
RTECS number:ES8120000
BRN number:1747101
PubChem number:24885171
Physical property data
1. Properties: colorless liquid[1]
2. Melting point (℃): -91.5[2]
3. Boiling point (℃): 164~165[3]
4. Relative density (water=1): 0.871[4]
5. Relative vapor density (air=1): 5.0[5]
6. Saturated vapor pressure (kPa): 1.73 (55℃ )[6]
7. Heat of combustion (kJ/mol): -4839.6[7]
8. Alcohol/water partition coefficient: 2.06[8]
9. Flash point (℃): 53 (OC)[9]
10. Explosion upper limit (%): 6.1[10]
11. Explosion lower limit (%): 1[11]
12. Solubility: Insoluble in water, miscible in ethanol and ether. [12]
13. Refractive index at room temperature (n25): 1.4029
14. Heat of generation (KJ/mol ): 539.7
15. Specific heat capacity (KJ/(kg·K), constant pressure): 1.92
16. Liquid phase standard hot melt (J·mol-1 ·K-1): 286.2
Toxicological data
1. Skin/eye irritation
Standard Draize test: rabbit, skin contact: 500mg/24H, severity of reaction: moderate.
2. Acute toxicity:
Rat intraperitoneal LD50: 2300mg/kg; Mouse intraperitoneal LC50: 8900mg/kg; Rabbit oral LD50: 9520mg/kg; Rabbit intraperitoneal LC50: 9520mg/kg; Skin contact LD50: >5mg/kg
3. Acute toxicity[13] LD50: 9520mg/kg (rabbit Oral)
4. Irritation [14] Rabbit transdermal: 500mg (24h), severe irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[15] This substance may be harmful to the environment and is harmful to the environment. Bodies of water should be given special attention.
Molecular structure data
1. Molar refractive index���40.88
2. Molar volume (cm3/mol): 164.0
3. Isotonic specific volume (90.2K) 375.1
4. Surface tension (dyne/cm): 27.3
5. Dielectric constant:
6. Dipole moment (10-24cm 3):
7. Polarizability: 16.20
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 89.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. The chemical properties are relatively stable, but it can be hydrolyzed in sodium hydroxide alcohol solution to generate butyric acid and butanol. About 90% hydrolysis occurs when heated at 60°C for 10 minutes.
2. Stability[16] Stable
3. Incompatible substances[17] Strong oxidants, strong bases, strong acids
4. Polymerization hazards[18] No polymerization
Storage method
Storage Precautions[19] Stored in a cool, ventilated warehouse. The storage temperature should not exceed 37°C. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the esterification of butyric acid and butanol. Add butyric acid, butanol and a small amount of sulfuric acid to the reaction pot, heat to reflux and dehydrate for about 4-5 hours, then neutralize and wash with alkali solution, fractionate under reduced pressure, and collect the 112°C (14.6kPa) fraction to obtain the finished product.
2. It is obtained by esterification of butyric acid and butanol under the catalysis of sulfuric acid, followed by washing, drying and distillation.
Refining method: Contains impurities such as free butyric acid and butanol. If butanol produced by fermentation is used as raw material, it also contains butyrate esters of isobutanol, pentanol and hexanol. During refining, wash with sodium bicarbonate and water, dry with anhydrous copper sulfate or sodium carbonate and then rectify.
3. Butyl butyrate can be generated by passing n-butanol vapor through manganese dioxide or zinc oxide catalyst at 400°C.
![]()
4. Preparation method:
p>
In a reaction bottle equipped with a stirrer and a reflux condenser, add 50 mL of n-butyraldehyde (2) and 5.0 mL of a carbon tetrachloride solution containing 2.5 g of aluminum isopropoxide. The reaction is exothermic and the temperature is rapid. Raise to boil. After the reaction is completed, dilute hydrochloric acid is used in sequence. Wash with water, dry over anhydrous magnesium sulfate, and fractionate. Collect the fractions at 164-166°C to obtain compound (1) ①, with a yield of 60%. Note: ① If the reaction is controlled below 20°C, the yield of butyl butyrate can reach 94%. When aluminum tert-butoxide is used as a catalyst and the reaction is carried out at low temperature, the yield is 90%. [21]
Purpose
1. Used in organic synthesis as solvents and perfumes. Butyl butyrate is used as a solvent, used in nitrocellulose, shellac, coumarone resin and paint solvents; used as a spice, it mainly contains apple, pineapple and other flavors.
2. Used to prepare banana, pineapple, apple and other fruity food flavors and whiskey flavors, and can also be used to flavor cream flavors. Generally, the dosage used in chewing gum is 150-1500 mg/kg; in candies, 24 mg/kg; in cold drinks, 22 mg/kg; in baked goods, 22 mg/kg; and in beverages, 8.6 mg/kg.
3. Used in small amounts in fruity daily flavors. It is widely used in pineapple, pear, peach, apple and other food flavors, whiskey flavors and tobacco flavors. The dosage in candies is 24×10-6, and the dosage in chewing gum is (150~1500)×10-6.
4. Used as a spice, used to prepare edible, tobacco and soap flavors, and to blend apple, pineapple and other flavors. It can also be used as a solvent for spray paint resin and nitrocellulose.
5. Used as solvent, standard material for chromatographic analysis, and also used in organic synthesis. [20]