Butyl lactate
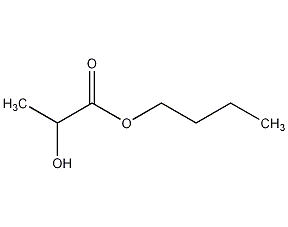

Structural formula
| Business number | 03RV |
|---|---|
| Molecular formula | C7H14O3 |
| Molecular weight | 146.17 |
| label |
n-butyl lactate, α-Hydroxybutylpropionate, Butyl-2-hydroxypropionate, n-Butyl lactate, α-Hydroxy propionic acid, Butyl-2-hydroxy propionate, CH3CH(OH)COOC4H9, dry cleaning fluid, adhesive, anti-skinning agent, spices, Multifunctional solvent |
Numbering system
CAS number:138-22-7
MDL number:MFCD00004519
EINECS number:205-316-4
RTECS number:OD4025000
BRN number:None
PubChem number:24857101
Physical property data
1. Properties: colorless and stable liquid. Mild smell. Hydrolyzes in the presence of acids and bases, but does not absorb water.
2. Density (g/mL, 20/4℃): 0.9837
3. Melting point (ºC): -43
4. Boiling point (ºC , normal pressure): 185
5. Refractive index (20ºC): 1.4217
6. Viscosity (mPa·s, 20ºC): 3.58
7. Flash point (ºC): 71
8. Vapor pressure (kPa, 75ºC): 1.33
9. Heat of evaporation (KJ/kg, 20ºC): 324.0
10. Body expansion coefficient (K-1): 0.00099
11. Solubility: Slightly soluble in water, miscible with hydrocarbons and oils. Can dissolve nitrocellulose, cellulose acetate, natural resin and synthetic resin, etc. It dissolves 4.0% in water at 20℃ and 3.4% at 25℃.
Toxicological data
1. Skin/eye irritation data: Rabbit skin contact: 500mg/24H moderate reaction
2. Acute toxicity data: Rat oral LD50: >5mg/kg; Rat subcutaneous LD50: 12mg/kg
Mouse subcutaneous LCLo: 11mg/kg; rabbit skin contact LD50: > 5mg/kg
3. A concentration of 7~11mg/kg in the air will cause headaches, mucous membrane irritation and coughing , occasional lethargy, nausea and vomiting, but no changes in blood and urine. ACGIH recommends a TLV of 5mg/kg. The product produced moderate irritation 1 day after application to rabbit skin under occlusive conditions. If a 1% concentration of petroleum jelly preparation is used for closed skin treatment on the human body,Sensitization will occur two days after the exposure test.
Ecological data
Molecular structure data
1. Molar refractive index: 37.74
2. Molar volume (cm3/mol): 145.4
3. Isotonic specific volume (90.2K): 349.4
4. Surface tension (3.0 dyne/cm): 33.3
5. Polarizability (0.5 10-24cm3): 14.96
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.1
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 46.5
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 101
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. There are three isomers. It has a sweet, fruity aroma, is soluble in water, and is miscible in various paint solvents and vegetable oils.
2. Chemical properties: Butyl lactate is difficult to dissolve in water and not easy to hydrolyze. When air is introduced at 180°C for a long time, it is gradually oxidized to pyruvic acid. In the presence of copper-chromium catalyst, propylene glycol is generated during hydrogenation at 225°C and 15~20MPa. Reacts with ammonia to form lactamide and butanol.
Storage method
Seal and store in a cool and ventilated place.
Synthesis method
1. Obtained from the esterification of lactic acid and n-butanol. The esterification reaction is carried out in the presence of sulfuric acid. After the reaction is completed, the finished product is obtained by neutralization and distillation under reduced pressure. The esterification reaction can also be carried out in benzene or toluene solvents. The content of industrial product butyl lactate is ≥95%. Raw material consumption quota: lactic acid (80%) 1000kg/t, butanol 650kg/t.
Purpose
1. Used as a solvent for nitrocellulose paint, printing ink, natural and synthetic resins, etc. Also used in dry cleaning fluids, adhesives, anti-skinning agents and spices. It is a high boiling point solvent, used in natural resins, synthetic resins, paints, and printing inks.
2.Cosmetic solvents. Mainly used as the main solvent for cosmetics such as nail polish. It has excellent solubility for film-forming agents such as nitrocellulose, and co-solvents are usually added to adjust the viscosity and evaporation rate.