Cholic acid Cholic acid
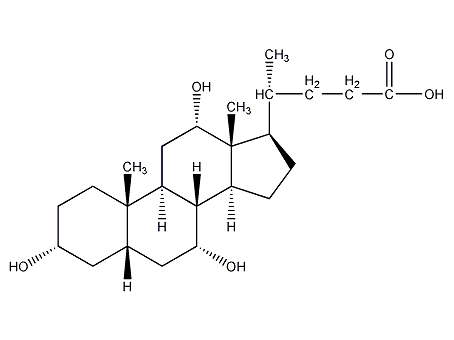

Structural formula
| Business number | 01S0 |
|---|---|
| Molecular formula | C24H40O5 |
| Molecular weight | 408.57 |
| label |
3α,7α,12α-Trihydroxy-5β-cholanic acid, cholanic acid, Sodium cholate; hyodeoxycholic acid; 3,7,12-trihydroxy-5β-cholanic acid, Lipoids |
Numbering system
CAS number:81-25-4
MDL number:MFCD00003672
EINECS number:201-337-8
RTECS number:FZ9350000
BRN number:2822009
PubChem number:24892356
Physical property data
1. Characteristics: This product exists in the bile of cattle, sheep and pigs. It is colorless flakes or white crystalline powder. The taste is sweet at first and then bitter.
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): Anhydrous melting point 198℃
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2 kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º ): [α] 20D is +37° (in ethanol)
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC ): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain OK
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Soluble in solutions of alkali metal hydroxides or carbonates.
Toxicological data
1. Acute toxicity
Mouse caliber LC50: 4950mg/kg; Mouse intravenous LC50: 330mg/kg;
2. Teratogenicity
Salmonella: 50mg/L;
Yeast: 93200 umol/L; Yeast: 400 mg/L;
Rat: 7560 mg/kg/6W
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 111.24
2. Molar volume (cm3/mol): 344.8
3. Isotonic specific volume (90.2K ): 920.9
4. Surface tension (dyne/cm): 50.8
5. Polarizability (10-24cm3): 44.09
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 3.6
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 4
5. Topological molecular polar surface area (TPSA): 98
6. Number of heavy atoms: 29
7. Surface charge: 0
8. Complexity: 637
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters : 11
11. Uncertain number of atomic stereocenters: 0
12. Determined number of chemical bond stereocenters: 0
13. Uncertain chemical bond formation Number of structural centers: 0
14. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
Multi-layer kraft paper bags wrapped in plastic bags are sealed and packaged with light or sealed in brown glass bottles. Store in low temperature dry warehouse.
Synthesis method
1. There are ethanol crystallization method and ethyl acetate separation method. The ethyl acetate separation method uses ethyl acetate to dissolve and remove deoxycholic acid, and then further purifies it with ethanol to obtain the product. The production process of the ethanol crystallization method is:
Hydrolysis of cattle and sheep bile ↓Acidification with sodium hydroxide ↓Crude bile acid sulfate
Dissolution, crystallization and recrystallization ↓Refined bile acid with ethanol
Add 1/10 amount of sodium hydroxide to the bile of cattle and sheep, heat to boiling, and hydrolyze for 18 hours. The hydrolyzate is cooled, the supernatant is poured out, and the lower layer is filtered. The supernatant and filtrate are combined, and 30% sulfuric acid is added to make the pH = 2 to 3. At this time, crude bile acid floats in the liquid layer. Take the supernatant, add an equal amount of water and boil for 10 to 20 minutes to precipitate into granules, rinse with water repeatedly until neutral, and dry at 50 to 60°C to obtain crude bile acid. Take the crude bile acid, mash it, add 0.75 times 75% ethanol, heat and stir, reflux until the solid is fully dissolved, and then crystallize it at 0 to 5°C. The crystals are suction filtered or centrifuged to dryness, washed with a small amount of 80% ethanol, and dried to obtain crude bile acid crystals. Take the crude crystals of cholic acid, add 4 times the amount of 95% ethanol and 4% to 5% activated carbon, heat, stir, and reflux until fully dissolved, then filter while hot. The filtrate is concentrated to about 1/4 volume and placed at 0 to 5°C for crystallization. Separate the crystals, wash with a small amount of 95% ethanol, and dry to obtain refined bile acid.
Purpose
1. This product can be used as an emulsifier.