cis-jasmone
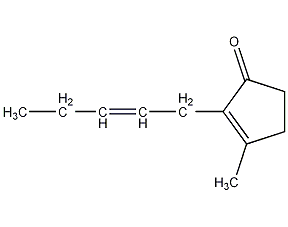

Structural formula
| Business number | 053K |
|---|---|
| Molecular formula | C11H16O |
| Molecular weight | 164.25 |
| label |
3-Methyl-2-(2′-pentenyl)-2-cyclopentenone, 3-Methyl-2-(2′-pentenyl)-2-cyclopentyl ketene, artificial flavors |
Numbering system
CAS number:488-10-8
MDL number:MFCD00001402
EINECS number:207-668-4
RTECS number:GY7301000
BRN number:1907713
PubChem number:24881767
Physical property data
1. Properties: light yellow oily liquid
2. Density (g/ m3, 25/4℃): 0.94
3. Boiling point (ºC, normal pressure): 134-135
4. Refractive index (n/22D): 1.498
5. Flash point (ºF): 225
6. Solubility: slightly soluble in water
Toxicological data
1. Skin/eye irritation data: Standard Draize test rabbit direct contact with skin: 500 mg/24 HREACTION SEVERITY: moderate
2. Acute toxicity: rat oral LD50: 5gm/kg, except No detailed description except the lethal dose;
Rabbit direct skin contact LD50: >5gm/kg, no detailed description except the lethal dose
Ecological data
Generally not hazardous to water, do not discharge material into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 50.77
2. Molar volume (cm3/mol): 175.1
3. Isotonic specific volume (90.2K ): 413.9
4. Surface tension (dyne/cm): 31.1
5. Polarizability (10-24cm3):20.12
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 10
6. Topological molecular polar surface area (TPSA): 17.1
p>
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 233
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Use and store according to specifications. It will not decompose and avoid contact with oxides. Light yellow oily liquid. Elegant jasmineFragrance and celery seed aroma. Slightly soluble in water, soluble in ethanol, ether, carbon tetrachloride and grease. Natural products are found in jasmine oil, neroli oil, bergamot oil, etc.
Storage method
Seal and store in a ventilated, dry environment
Synthesis method
Jasmone is a component of essential oils such as jasmine oil, narcissus oil, and neroli oil. There are many methods for industrial synthesis, such as using 3-hexenol as raw material. For example, methyl vinyl ketone is used as raw material, and hydrogen bromide is added to obtain 2-bromoethyl ketone to form a cyclic ketal. The metal compound of acetylene is condensed in a dihydroxy acid solution to form acetylene ketal. Then react with the p-toluenesulfonate of cis-2-penten-1-ol, and treat the product with perchloric acid to form cis-8-(+)-en-5-yn-2-one, and then add Hydrogen makes 2,4-dione. Intramolecular condensation of 2,4-diketone in a dilute aqueous alkali solution gives cis-jasmonone. Another preparation method is to cyclize γ-ketoaldehyde and then introduce a methyl group to prepare jasmonone.
Purpose
Shunjasmone is one of the most valuable spices. Its aroma is very similar to that of jasmine flowers. It is one of the important fragrance components of jasmine oil and is used in high-end jasmine series chemical flavors. . Dihydrojasmone can be obtained by hydrogenation and reduction of jasmone. Dihydrojasmone is a slightly colored transparent liquid with a boiling point of 230°C, 102°C (0.67kPa). It has a strong floral fragrance and relatively stable chemical properties. It can be widely used in jasmine series flavors. The industrial production of dihydrojasmone is simple. There are many ways. In the presence of phosphoric acid, the temperature is controlled at 80°C and the pressure is 0.007-0.011MPa. Dihydrojasmonone can be obtained by cyclizing undecylenic acid.