Cyanoacetic acid
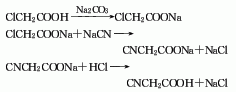
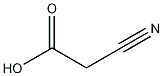
Structural formula
| Business number | 04J0 |
|---|---|
| Molecular formula | C3H3NO2 |
| Molecular weight | 85 |
| label |
None yet |
Numbering system
CAS number:372-09-8
MDL number:MFCD00002677
EINECS number:206-743-9
RTECS number:AG3675000
BRN number:506325
PubChem ID:None
Physical property data
1. Properties: white crystal, hygroscopic. [1]
2. Melting point (℃): 66~68[2]
3. Boiling point (℃) : 108 (2.0kPa) [3]
4. Saturated vapor pressure (kPa): 0.0133 (100℃) [4]
5. Heat of combustion (kJ/mol): -1249[5]
6. Octanol/water partition coefficient: -0.76[6]
7. Flash point (℃): 107.8[7]
8. Solubility: soluble in water, ethanol, ether, Slightly soluble in benzene, acetic acid and chloroform. [8]
Toxicological data
1. Acute toxicity[9] LD50: 1500mg/kg (rat oral)
2. Irritation No information yet
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[10] This substance is harmful to the environment and should be treated with special Pay attention to water pollution.
Molecular structure data
1. Molar refractive index: 17.42
2. Molar volume (cm3/mol): 66
3. Isotonic specific volume (90.2K ): 181.5
4. Surface tension (dyne/cm): 57
5. Dielectric constant: None available
6. Polarizability ( 10-24cm3): 6.9
7. Single isotope mass: 85.016378 Da
8. Nominal mass: 85 Da
9. Average mass: 85.0614 Da
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 61.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 98.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[11] Stable
2. Incompatible substances[12] Strong oxidizing agent, strong reducing agent, strong acid, strong alkali
3. Conditions to avoid contact[13] Moisture
4. Polymerization hazard[14] No polymerization
5. Decomposition products[15] Cyanide
Storage method
Storage Precautions[16] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, reducing agents, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Using chloroacetic acid, sodium carbonate, and sodium cyanide as raw materials, it is obtained by neutralization, cyanidation, and acidification with hydrochloric acid. Add the sodium carbonate suspension to chloroacetic acid, and neutralize it through circulation below 45°C until pH=7.5-8. After the neutralization is completed, a sodium chloroacetate liquid is obtained. Add the neutralized liquid to the sodium cyanide solution and perform cyanidation at a temperature of 105-115°C. Keep the temperature for 2-3 minutes to obtain sodium cyanoacetate. Then cool the temperature to below 50°C and put the sodium cyanoacetate into a dehydration pot with hydrochloric acid. Acidification, and finally dehydration under reduced pressure, the finished product of cyanoacetic acid is obtained. Raw material consumption quota: chloroacetic acid (95%) 1240kg/t, sodium cyanide (96%) 710kg/t.
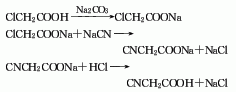
Purpose
1. Organic synthesis intermediates, mainly used in the synthesis of cyanoacetate esters, and also used in the production of alpha methyl cyanoacrylate and n-butyl ester (medical adhesives), vitamin B6, caffeine, barbiturates, etc.
2. Used in organic synthesis and used in medicine to produce caffeine. [17]