diethyl maleate
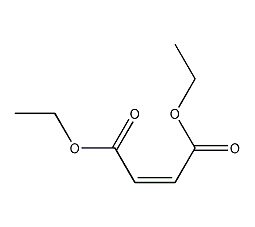

Structural formula
| Business number | 03TP |
|---|---|
| Molecular formula | C8H12O4 |
| Molecular weight | 172 |
| label |
None yet |
Numbering system
CAS number:141-05-9
MDL number:MFCD00009191
EINECS number:205-451-9
RTECS number:ON1225000
BRN number:1100825
PubChem number:24882313
Physical property data
1. Properties: colorless and transparent liquid.
2. Boiling point (ºC, 101.3kPa): 223
3. Melting point (ºC): -8.8
4. Relative density (25℃, 4 ℃): 1.001486.1
5. Relative vapor density (g/mL, air=1): 5.93
6. Refractive index (25ºC): 1.4383
7. Viscosity (mPa·s, 25ºC): 3.14
8. Flash point (ºC): 93
9. Heat of vaporization (KJ/ mol): 52.3
10. Vapor pressure (kPa, 30ºC): 0.30
11. Volume expansion coefficient (K-1, 20~30ºC) : 0.00094
12. Solubility: Miscible with a variety of organic solvents, partially soluble in benzene and chloroform. It dissolves 1.4% in water at 30℃; water dissolves 1.9% in diethyl maleate. It forms an azeotropic mixture with 88.2% water, with an azeotropic point of 99.65°C.
13. Relative density (20℃, 4℃): 1.0662
14. Refractive index at room temperature (n20): 1.4416
15. Liquid phase standard hot melt (J·mol-1·K-1): 296.2
Toxicological data
1. Acute toxicity: Rat oral LD50: 3200 mg/kg; Rat transdermal LD50: 5000 mg/kg;
Rabbit skin LD50: 530 mg; Rat abdominal LD50: 3070 mg/kg;
Rabbit transdermal LD50: 4 mL/kg.
2. It is slightly irritating to the skin and eyes. Rats did not die after inhaling saturated steam for 8 hours.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 42.71
2. Molar volume (cm3/mol): 161.1
3. Isotonic specific volume (90.2K ): 387.1
4. Surface tension (dyne/cm): 33.2
5. Polarizability (10-24cm3): 16.93
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 0.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 6
5. Topological molecular polar surface area (TPSA): 52.6
6. Number of heavy atoms: 12
7. Surface charge: 0
8. Complexity: 164
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters: 0
11. The number of uncertain atomic stereocenters: 0
12. The number of determined chemical bond stereocenters: 1
13. The number of uncertain chemical bond stereocenters: 0
14. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure. Incompatible materials: oxidizing agents, reducing agents, acids, alkalis. Can burn, please pay attention to fire source when using and storing. Prevent inhalation of vapors and avoid contact with skin.
Storage method
Store in a cool, dry and ventilated place. Pay attention to fire sources when using and storing.
Synthesis method
1. It is produced by the esterification of maleic anhydride and ethanol in the presence of sulfuric acid; it can also be obtained by exchange conversion using cation exchange resin as a catalyst. The content of diethyl maleate in industrial products is ≥98%, and each ton of product consumes 585kg of maleic anhydride (95%) and 604kg of ethanol (95%).
2. Its preparation method is mainly prepared by esterification of maleic anhydride and ethanol in the presence of sulfuric acid. This process includes two types: normal pressure phenyl esterification and negative pressure phenyl-free esterification.
(1) Esterification of benzene under normal pressure
Add a certain amount of benzene and ethanol into the esterification reaction pot, add maleic anhydride, add concentrated sulfuric acid dropwise under stirring, and heat with jacketed steam , causing the reactants to undergo esterification reaction at about 75°C. The generated water, benzene, and ethanol are removed through three-way azeotropic distillation, and the benzene and ethanol liquids in the upper layer are refluxed into the reaction pot. About 13 to 14 hours later, when the temperature at the top of the distillation tower rises to 68.2°C and the water level in the lower layer of the separator no longer rises, it indicates that all the water in the reaction pot has been evaporated and the esterification reaction is completed. Stop reflux, continue distillation to 95-100°C, and steam out benzene and ethanol. Cool to about 50°C, neutralize with 5% sodium carbonate aqueous solution, wash with water and remove residual benzene and ethanol in a vacuum to obtain the product diethyl maleate.
(2) Negative pressure phenyl-free esterification
Maleic anhydride and ethanol are esterified under the action of sulfuric acid, and the ethanol and the water generated by the reaction are taken out in a gaseous state under a certain vacuum and temperature. The ethanol is then separated through a fractionation tower and refluxed for esterification to complete the reaction. This method can shorten the reaction cycle, improve yield and product quality, and improve the operating environment. Most domestic production plants use this method.
In addition, cation exchange resin can also be used as a catalyst for exchange conversion to produce diethyl maleate.
Refining method: wash with dilute potassium carbonate solution, dry with anhydrous potassium carbonate or sodium sulfate, and then distill under reduced pressure.
Purpose
Diethyl maleate is a polymer compound monomer and an intermediate for pesticides, medicines, spices, and water quality stabilizers (organic polycarboxylic acid phosphonic acid compounds). Mainly used in the production of organophosphorus pesticide malathion (marathion). Used as polymer monomer and plasticizer for synthetic resin. Also used as pesticides, fungicides, anti-rust additives, etc.