Dimethyl malonate Dimethyl malonate
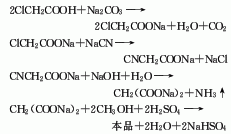
Structural formula
| Business number | 02XJ |
|---|---|
| Molecular formula | C5H8O4 |
| Molecular weight | 132.11 |
| label |
dimethyl malonate, Methyl malonate, dimethyl caroteate, Dimethylmalonate, Propanedioic Acid Dimethyl Ester, Dimethyl Ester Of Malonic Acid, Dimethyl Propanedioate, Dimethyl1,3-Propanedioate, Dimethylpropanedioate, Malonic Acid,Bis-Methyl Ester, Malonic Acid Dimethyl Ester, Methyl Malonate, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:108-59-8
MDL number:MFCD00008460
EINECS number:203-597-8
RTECS number:None
BRN number:774261
PubChem number:24854457
Physical property data
1. Properties: colorless liquid
2. Density (g/mL, 25℃): 1.156
3. Relative vapor density (g/mL, air=1 ):>1
4. Melting point (ºC): −62
5. Boiling point (ºC, normal pressure): 181.4
6. Relative density ( 25℃, 4℃): 1.144730
7. Refractive index (D20): 1.4135
8. Flash point (ºC): 90
9. Relative density (20℃, 4℃): 1.1528
10. Refractive index at room temperature (n 20): 1.4135
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in organic solvents such as alcohol and ether, slightly soluble in water.
Toxicological data
1. Skin/eye irritation
Standard Draize test: rabbit, skin contact: 500mg/24H.
2. Acute toxicity: Rat oral LD50: 5331mg/kg; rabbit skin contact LD50: >5mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 28.76
2. Molar volume (cm3/mol): 118.0
3. Isotonic specific volume (90.2K ): 280.5
4. Surface tension (dyne/cm): 31.9
5. Polarizability (10-24cm3): 11.40
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 52.6
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 104
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides and alkali.
2.This product is slightly more toxic than diethyl malonate. Rat oral LD50: 5331mg/kg. Dimethyl malonate will hydrolyze in the body to produce malonic acid. Inhalation or skin contact should be avoided as much as possible. Wash immediately after contact.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging requires sealing and should be stored separately from oxidants and strong alkali. Mixed storage is strictly prohibited. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
This product is packed in iron drums, 200kg per drum. There are no special requirements for storage. Store and transport according to general chemical regulations
Synthesis method
Cyanide esterification method is often used. Neutralization of chloroacetic acid and sodium carbonate produces sodium chloroacetate, which is then cyanated with sodium cyanide to obtain sodium cyanoacetate. Sodium cyanoacetate is hydrolyzed into sodium malonate, and then esterified with methanol in the presence of sulfuric acid to obtain dimethyl malonate, which is washed and distilled to obtain the finished product. Industrial products contain ester content ≥98%. Raw material consumption quota: chloroacetic acid 1120kg/t, sodium cyanide 551kg/t, methanol 955kg/t. The new process developed abroad is mainly based on the catalytic carbonylation method, which uses chloroacetate, carbon monoxide, and methanol as raw materials, and in the presence of a catalyst, dimethyl malonate is synthesized in one step. In contrast, the catalytic carbonylation process has advanced technology, but the process is complex and the reaction conditions are harsh, making industrialization difficult.
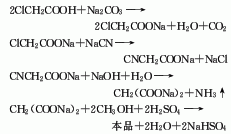
Purpose
Pharmaceutical intermediates. Sodium 2,5-bis(4,6-dimethoxypyrimidine-2-oxy)benzoate can be synthesized using thiourea, dimethyl malonate and resorcinol as the main raw materials.