diphenyl sulfide
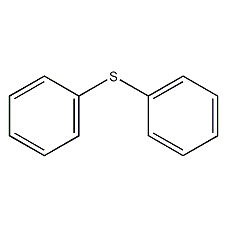
Structural formula
| Business number | 03SG |
|---|---|
| Molecular formula | C12H10S |
| Molecular weight | 186.27 |
| label |
diphenylsulfide, Phenyl sulfide, Diphenyl sulfide, thiobenzene, benzene sulfide, diphenyl sulfide, Phenyl sulfide, aromatic sulfur compounds |
Numbering system
CAS number:139-66-2
MDL number:MFCD00003064
EINECS number:205-371-4
RTECS number:SX2275000
BRN number:1907932
PubChem number:24898423
Physical property data
1. Properties: colorless or light yellow liquid with foul odor.
2. Relative density: 1.1185
3. Melting point (ºC): -40
4. Refractive index: 1.635
5 . Solubility: Easily soluble in ether, carbon disulfide, and benzene, soluble in hot alcohol, but insoluble in water.
6. Boiling point: 296ºC, 189ºC (666kPa)
Toxicological data
Acute toxicity data:
Rat oral LD50: 490uL/kg;
Rabbit skin LD50: 11300uL/kg;
Rabbit skin contact: Severe reaction at 10mg/24H.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 59.47
2. Molar volume (cm3/mol): 164.6
3. Isotonic specific volume (90.2K): 429.8
4. Surface tension (3.0 dyne/cm): 46.4
5. Polarizability (0.5 10-24cm3): 23.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 25.3
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 116
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Poisonous. Can cause serious injury or death if swallowed, inhaled or absorbed through skin contact. Suitable protective clothing should be worn.
Storage method
Should be sealed and stored in a cool place.
Synthesis method
Mix 858g anhydrous benzene and 464g aluminum trichloride, cool to 10°C, and add a solution of 405.1g sulfur chloride and 390g benzene while stirring. The reaction begins immediately,Hydrogen chloride is released and a yellow viscous aluminum trichloride complex is precipitated. After the reaction is completed, the reaction mixture is iced out. Separate the benzene layer, evaporate the benzene and cool the dark oil to 0°C, and filter out the precipitated sulfur. Then dissolve the oil in methanol, cool to 0°C, and filter out the precipitated sulfur. After recovering the methanol, the methanol solution continues to evaporate a small amount of low boiling point fractions. Then collect the 155-170°C (2.39kPa) fraction, which is the crude product. The crude product is heated with zinc powder and sodium hydroxide, washed with water, dried, and finally distilled under reduced pressure to obtain 450-464g of diphenyl sulfide.
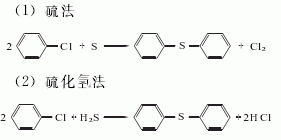
1. Chlorobenzene method: Using chlorobenzene as the starting material, there are methods of reacting with sulfur and reacting with hydrogen sulfide.

2.Diphenyl disulfide method
Dissolve a certain amount of diphenyl disulfide and iodobenzene in ethyl acetate and irradiate with a low-pressure mercury lamp. The product yield is 12%.
Purpose
Used as intermediates for pesticides, medicines and dyes. This product can be used to synthesize 2,4-dichlorophenol, which is used to synthesize herbicidal ether, 2,4-D, EPBP, poisonous powder, etc.; in medicine, it is used to synthesize thiobisdichlorophenol, etc.