Dithizone Dithizone
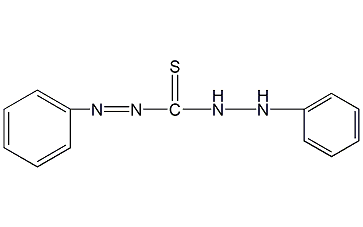
Structural formula
| Business number | 01AZ |
|---|---|
| Molecular formula | C13H12N4S |
| Molecular weight | 256.33 |
| label |
diphenylthiocarbazone, Diphenylthiodisemicarbazide, Phenylhydrazine thiocarbonyl azobenzene; lead reagent;, Diphenylthiocarbazone, Reagent |
Numbering system
CAS number:60-10-6
MDL number:MFCD00003025
EINECS number:200-454-1
RTECS number:LQ9450000
BRN number:748838
PubChem number:24893923
Physical property data
1. Properties: blue-black crystalline powder
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/ mL, air=1): Undetermined
4. Melting point (ºC): 165-169℃ (decomposition)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure ( kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil-water (octanol/water) partition coefficient relationship Value: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Easily soluble in chloroform and carbon tetrachloride, slightly soluble in alcohol, insoluble in water
Toxicological data
1. Acute toxicity: rat oral LD: >500mg/kg; mouse intraperitoneal LC50: 200mg/kg; mouse intravenous LC50: 56mg/kg
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 76.90
2. Molar volume (cm3/mol): 212.4
3. Isotonic specific volume (90.2K): 554.5
4. Surface tension (dyne/cm): 46.1
5. Polarizability (10-24cm3): 30.48
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecular polar surface area (TPSA): 48.8
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complex Degree: 281
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters : 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Covalent bond unit Quantity: 1
Properties and stability
1. With, Cu2+, Ag+, Au3+, Hg2+ , Fe2+ , Co2+ plasma forms a water-insoluble complex, which is easily dissolved by carbon tetrachloride and chloroform, etc. After solvent extraction, the complex usually appears golden or purple.
Storage method
This product should be sealed and stored in a cool place.
Synthesis method
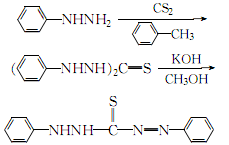
1. Prepare according to the following steps. 1. Preparation of phenylhydrazine dithiocarbamate salt. Dissolve 13g phenylhydrazine in 60ml ether, and add 5.2ml carbon disulfide dropwise. The resulting white precipitate was filtered off and washed with diethyl ether to obtain about 15 g. 2. Preparation of diphenylthiosemicarbazide. Place the above product in a beaker and heat it on a water bath at 96-98°C. After the material melts, it releases hydrogen sulfide and turns into a light yellow gel. If ammonia gas is released, remove the heat bath and cool with water. Cool again with ice. Then cool it with ice, add 15 ml of water and absolute ethanol, take it out from the ice bath, and heat it slightly while stirring. At this time, the gelatin becomes a crystalline product, filter it with suction, and wash it with ethanol to obtain about 7.5g. 3. Preparation of dithizone. Add the above-obtained diphenylthiosemicarbazide to the caustic potassium solution (6g potassium hydroxide dissolved in 60ml anhydrous methanol), reflux on a boiling water bath for 5 minutes, take it out and cool it in an ice bath. Filter, and the filtrate is acidified with sulfuric acid until the Congo red test paper changes color and a dark blue precipitate precipitates. After suction filtration, the precipitate is treated with caustic potassium solution and sulfuric acid again. After suction filtration, it is washed with water until no sulfate radicals exist. After drying at 40℃, about 3g of dithizone is obtained.
2.Cool the mixture of phenylhydrazine and toluene below 0℃, add carbon disulfide under stirring, and control the addition speed to maintain the reaction temperature at 60~70℃, leave it for more than 10 minutes, then heat to 90~95℃, the crystals will dissolve and the reaction will begin. When a large amount of crystals precipitate and the lead acetate reagent turns slightly brown, the reaction is completed. After cooling, it is filtered. The crystals are washed with cold toluene and ethanol in sequence. The white crystals obtained are
diphenylthiocarbazine. Then, add diphenylcarbazine to the methanol solution of potassium hydroxide, heat to reflux for 5 to 10 minutes, cool the ice water to about 30°C, filter out the insoluble matter, and add 0.5 mol/L sulfuric acid to the filtrate while stirring. The aqueous solution is until the solution is just acidic to the Congo Red test paper. The crystals obtained by filtration are washed with cold water, and then dissolved in 5% sodium hydroxide solution. Acidify with mol/L sulfuric acid until the Congo red test paper is just acidic. After the precipitation is complete, filter it. The filtered crystals are fully washed with ice water until the SO42- ions are qualified. After draining, dry at 40°C to obtain the finished product diphenylthiocarbazone. The process reaction is:

Purpose
1. Determination of trace amounts of complex indicator, and used to determine cobalt, copper, mercury, zinc and silver, etc.
2.Used as a chromogen for the photometric determination of lead, cobalt, copper, mercury, zinc and silver, etc., and also used as Metal ions cooperate with titration indicators. It can also be used as a metal ion extractant.