Ethanethiol Ethanethiol
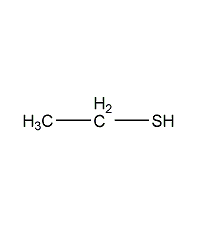
Structural formula
| Business number | 01HY |
|---|---|
| Molecular formula | C2H6S |
| Molecular weight | 62.13 |
| label |
Ethyl sulfide; ethyl mercapto;, Ethanethiol, Ethyl thioalcohol, aliphatic sulfur compounds, Alcohols |
Numbering system
CAS number:75-08-1
MDL number:MFCD00004887
EINECS number:200-837-3
RTECS number:KI9265000
BRN number:773638
PubChem number:24855097
Physical property data
1. Properties: Colorless and volatile liquid with a strong and unpleasant odor.
2. Density (g/mL, 25/4℃): 0.8315
3. Relative vapor density (g/mL, air=1): 2.14
4. Melting point (ºC): -147.89
5. Boiling point (ºC, normal pressure): 35
6. Refractive index (20ºC): 1.4310
7. Flash point (ºC): -45
8. Viscosity (mPa·s, 20ºC): 0.293
9. Fire point (ºC): 299
10. Critical temperature (ºC): 225.5
11. Critical pressure (KPa): 5.32
12. Vapor pressure (kPa, 20ºC): 58.928
13. Explosion upper limit (%, V/V): 18.2
14. Explosion lower limit (%, V/V): 2.8
15. Solubility: Slightly soluble Slightly soluble in water, the solubility in water is 1.5% (weight ratio) at 20°C, and it can dissolve 6.76g per liter of water at 25°C. It can form hydrates with water, and the hydrates will crystallize when the temperature is lower than 10℃. Easily soluble in alkaline water and organic solvents such as ethanol and ether.
Toxicological data
This product is highly toxic. Inhalation of large amounts will cause lowered blood pressure, difficulty breathing, and symptoms such as vomiting, diarrhea, and hematuria. Extremely toxic to aquatic life. May cause adverse consequences for the aquatic environment.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 19.21
2. Molar volume (cm3/mol): 75.5
3. Isotonic specific volume (90.2K ): 163.5
4. Surface tension (dyne/cm): 21.9
5. Polarizability (10-24cm3): 7.61
Calculate chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 0.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6.Topological molecular polar surface area (TPSA): 0
7, Number of heavy atoms: 3
8, Surface charge: 0
9, Complexity: 2.8
10. The number of isotope atoms: 0
11. The number of determined atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product is highly toxic and can cause a drop in blood pressure and difficulty breathing in dogs at levels below 1%. Inhalation of large amounts by the human body can cause lowered blood pressure, difficulty breathing, and symptoms such as vomiting, diarrhea, and hematuria. Those with mild symptoms can gradually recover if they leave the scene in time; those with severe symptoms can be sent to the hospital for treatment immediately. The maximum allowable concentration in the air is 0.5×10-6. The production equipment should be sealed, the operation site should be forced to ventilate, and the operators should wear protective equipment.
2. Exist in smoke.
Storage method
1. Store sealed in a cool, dry and ventilated place. Store and transport according to regulations for flammable and toxic substances.
2. This product is generally used as an intermediate and is not sold as a commodity.
Synthesis method
1. Obtained from the reaction of sodium ethyl sulfate and sodium hydrosulfide. The sodium ethyl sulfate used in this method is prepared from absolute ethanol and fuming sulfuric acid. The total yield is 60%-65% (based on absolute ethanol). This method is relatively mature in China, but its disadvantages are long route, low yield, and high requirements for raw materials.
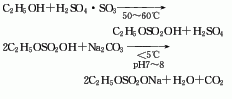
![]()
2. Obtained from the reaction of ethyl chloride and sodium hydrosulfide. The yield can reach more than 80% (based on chloroethanol).
![]()
3. Made of ethanol (or ethylene ) and hydrogen sulfide through gas phase catalytic reaction. The reaction is carried out under normal pressure. The catalyst uses activated alumina as a carrier and is impregnated with tungstic acid or sodium tungstate. The reaction temperature is 360-380°C. The yield of ethyl mercaptan (calculated as ethanol) can reach 70%-79%. 4. Laboratory preparation can be made from the reaction of thiourea and ethyl bromide.
![]()
4.Use thiourea and ethyl bromide as raw materials, heat and reflux in the solvent methanol for 3 to 4 hours. After the reaction is completed, recover the methanol, and then add 10% sodium hydroxide , continue to reflux for 4 hours, then cool to 5°C, slowly add 1:2 dilute sulfuric acid for acidification, and evaporate ethyl mercaptan under constant stirring. The reaction process is:
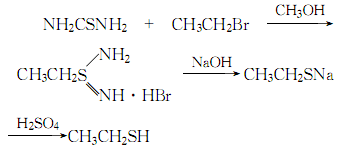
The crude ethyl mercaptan obtained is first washed with water to remove sodium hydroxide and sulfuric acid, then dried with anhydrous sodium sulfate to remove water, and finally fractionated and collected at 34-37°C The fraction is the finished product.
5. Using ethanol (or ethylene) and hydrogen sulfide gas as raw materials, the ratio is 1:3 (molar ratio), in the presence of activated alumina catalyst impregnated with tungstic acid (or sodium tungstate) Carry out the reaction and control the appropriate feeding rate [130g ethanol/(L catalyst·h)], the reaction temperature is 360~380℃, and the reaction pressure is normal pressure:
![]()
Excess sulfidation during the reaction Hydrogen gas can be recycled. The product ethyl mercaptan generated by the reaction should be captured at low temperature by freezing. After drying to remove the water, it can be fractionated and the 34-37°C fraction can be collected.
Purpose
1. Ethyl mercaptan is an important pesticide intermediate, used in the production of organophosphorus pesticides isopropylphos, phorate, acetate, systemic phosphorus, methyl systemic phosphorus, etc. It can also be used to produce antibacterial agent 401. When the concentration of ethyl mercaptan in the air is only one part per 50 billion, its odor can be detected, so it can be used as a warning agent and odorizer for natural gas and petroleum gas (the odor is detected at a concentration of 0.00019 mg/L can be smelled). Ethyl mercaptan can also be used in organic synthesis such as medicine.
2.Used in organic synthesis. Used as antioxidant, natural gas and petroleum gas warning agent.