Ethyl 3-methyl-3-phenyloxiranecarboxylate Ethyl 3-methyl-3-phenylglycidate
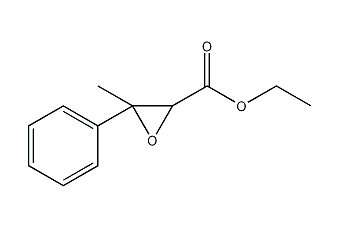

Structural formula
| Business number | 01M8 |
|---|---|
| Molecular formula | C12H14O3 |
| Molecular weight | 206.24 |
| label |
Hexadecylaldehyde, Ethyl methylphenylglycidate, 3-Methyl-3-phenylglycidyl ethyl ester, 2-Benzyl-2,3-cyclobutyric acid ethyl ester, B-Phenyl epoxybutyrate ethyl ester, strawberry aldehyde, Methylphenylglycidyl ethyl ester, Amaranth aldehyde, 2,3-epoxy-3-phenyl-butanoicacythylester, 3-methyl-3-phenyl-oxiranecarboxylicacidethylester |
Numbering system
CAS number:77-83-8
MDL number:MFCD00022338
EINECS number:201-061-8
RTECS number:MW5250000
BRN number:12299
PubChem number:24901091
Physical property data
1. Properties: colorless or light yellow transparent liquid
2. Density (g/mL, 25/4℃): 1.086-1.112
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): Uncertain
5. Boiling point (ºC, normal pressure ): 272-275℃
6. Boiling point (ºC, 5.2kPa): 147-149℃ (1.6KPa)
7. Refractive index: 1.504-1.513
8. Flash point (ºC): 110℃
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Uncertain
17. The upper explosion limit (%, V/V): Uncertain
18. The lower explosion limit (%, V/V): Uncertain
19. Solubility: Soluble in 3 times the volume of 60% ethanol, soluble in ether and chloroform, and can be mixed with diethyl phthalate, benzyl It is miscible with benzoic acid and almost insoluble in glycerin and water.
Toxicological data
1. Acute toxicity
Rat caliber LD50: 5470mg/kg; pig caliber LDLO: 4050 mg/kg
2. Other multi-dose toxicity data
Rat caliber LD50: 182 mg/kg/2Y-C
3. Teratogenicity
Hamster.Nest: 50mg/L; Hamster ovary: 16mg/L;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 55.46
2. Molar volume (cm3/mol): 181.0
3. Isotonic specific volume (90.2K): 454.6
4. Surface tension (dyne/cm): 39.7
5. Polarizability (10-24cm3): 21.98
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 38.8
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 245
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 2
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
Stored in a cool, dry place and not stacked in the open.
Synthesis method
1. This product is not artificially synthesized. It is produced by the condensation of acetophenone and ethyl chloroacetate in the presence of sodium ethoxide, that is, through the Claisen reaction. Based on acetophenone, the yield is about 40%.
2.From acetophenone and ethyl chloroacetate in sodium ethoxide or sodium amide It is obtained by condensation in the presence of water, neutralization, water washing, separation and vacuum distillation.
Purpose
1. This product has a strong bayberry fragrance and is usually used as a raw material for blending bayberry essence after dilution. It is also used in cosmetics. When blending flower essence stains for cosmetics such as roses, hyacinths and cyclamen, Adding a small amount of this product can produce special effects.
2.Used to prepare fruit-based food flavors such as cherries, grapes, apples, and strawberries. The amount used in gum candies is 470mg/kg; in soft drinks, it is 5.6mg/kg; 21mg/kg in candies; 18mg/kg; 13mg/kg in pudding; 6.7mg/kg in cold drinks span>.