Ethyl acetoacetate
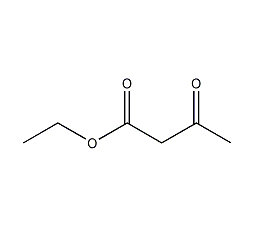

Structural formula
| Business number | 03UM |
|---|---|
| Molecular formula | C6H10O3 |
| Molecular weight | 130.14 |
| label |
Ethyl butylacetate; ethyl acetoacetate;, Acetoacetic ester, Diacetic ester, Ethy-3-oxobutanoate, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:141-97-9
MDL number:MFCD00009199
EINECS number:205-516-1
RTECS number:AK5250000
BRN number:385838
PubChem ID:None
Physical property data
1. Properties: colorless liquid with aromatic smell.
2. Boiling point (ºC, 101.3kPa): 181
3. Melting point (ºC, ketone form): -39
4. Melting point (ºC, Enol form): -44
5. Relative density (g/mL, 25/4ºC): 1.02126
6. Relative vapor density (g/mL, air=1) : 4.5
7. Refractive index (20ºC): 1.4194
8. Viscosity (mPa·s, 25ºC): 1.5081
9. Flash point (ºC , closed): 84.4
10. Ignition point (ºC): 295
11. Heat of combustion (KJ/mol, 25ºC): 3162.7
12. Specific heat capacity (KJ/(kg·K), 24.5ºC, constant pressure): 1.92
13. Critical temperature (ºC): 400
14. Conductivity (S/m, 25ºC ): 1×10-7
15. Vapor pressure (kPa, 40~41ºC): 0.27
16. Thermal conductivity (W/( m·K), 30ºC): 0.1558
17. Solubility: Slightly soluble in water, soluble in organic solvents. Dissolves 12% in water at 25°C; water dissolves 4.9% in ethyl acetoacetate.
18. Relative density (20℃, 4℃): 1.0282
19. Liquid phase standard hot melt (J·mol-1·K-1): 251.4
20. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3228.5
21. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -561.77
Toxicological data
1. Acute toxicity: Rat oral LD50: 3980 mg/kg; mouse oral LC50: 5105 mg/kg
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 31.65
2. Molar volume (cm3/mol): 128.1
3. Isotonic specific volume (90.2K ): 300.6
4. Surface tension (dyne/cm): 30.2
5. Polarizability (10-24cm3): 12.54
�50g (55mL, 0.57mol), reinstall the reflux condenser, the reaction starts immediately, and hydrogen bubbles are generated. If the reaction is slow, heat it appropriately. After a violent reaction, slowly heat and maintain a slight boil until the sodium metal reacts completely, which takes about 1.5 hours to produce an orange-red solution. After cooling slightly, add 50% acetic acid solution with stirring until it becomes weakly acidic, and all the solid matter is dissolved. Transfer the reactants into a separatory funnel, add an equal volume of saturated sodium chloride solution, shake thoroughly, separate the organic layer, and dry over anhydrous sodium sulfate. Filter, first evaporate the unreacted ethyl acetate, then fractionate under reduced pressure, collect the fraction at 100°C/10.66kPa, and obtain 12-14g of compound (1), with a yield of 42%-49% (based on the metal sodium used) . [1]
Purpose
1. Ethyl acetoacetate is an important raw material for organic synthesis. It is used in the production of pesticides to synthesize α-chloroethyl acetoacetate, the intermediate of the organophosphorus insecticide pyrimidinox, and the intermediate of pyrimidinoxphos. The body 2-methoxy-4-methyl-6-hydroxypyrimidine, the intermediate 2-isopropyl-4-methyl-6-hydroxypyrimidine of diazinon, and the carbamate insecticide pirimicarb, The fungicide cyprodinil, the herbicide ethylpyridinate, the rodenticides fenacetate, warfarin, etc. are also new varieties of fungicides such as cyprodinil, difulmetorim, and fupyram It is an intermediate of amine (furammetpyr) and the plant growth regulator cintofen. In addition, ethyl acetoacetate is used as a solvent for paints and analytical reagents. It is also widely used in medicine, plastics, dyes, perfumes, varnishes and additives. and other industries.
2. It is occasionally used in cosmetic flavors such as gardenia to give wine a fruity aroma or an elegant non-floral top aroma. It can be used in toilet water or liquids to give ethanol a rounded feel. It is widely used in food flavors and can be used in many fruity or wine flavors, such as apples, apricots, peaches, cherries, berries and rice wine, brandy, liquor, whiskey, rum and other food and wine flavors. GB 2760-96 stipulates the edible spices allowed to be used. It is widely used in the preparation of strawberry, apple, apricot, cherry, peach and other fruit-type and wine-type (rum, whiskey, etc.) flavors.
3. Ethyl acetoacetate is widely used in organic synthesis. For example, it can be used to synthesize heterocyclic compounds such as pyridine, pyrrole, pyrazolone, pyrimidine, purine and cyclic lactone. It is also widely used in drug synthesis. For example, ethyl acetoacetate and resorcinol are cyclized to obtain 4-methyl-7-hydroxycoumarin, which is an anti-allergic drug scale intermediate. The condensation of ethyl acetoacetate with benzyl chloride yields ethyl α-acetyl phenylpropionate, which is an intermediate for the antitussive drug methicillin. Ethyl acetoacetate is condensed with benzoyl chloride to obtain ethyl benzoacetate, which is an intermediate of the central stimulant drug lobeline hydrochloride. The antithyroid drug methylthiouracil (an intermediate of the coronary artery dilator dipyridamole) is produced by cyclizing ethyl acetate with thiourea. The particularity of the EAA structure determines that it has both the properties of a ketone and the reaction characteristics of an enol, and its chemical properties are very active. It is widely used in medicine, dyes, pesticides and other fields, as well as in food additives and flavors and fragrances. The biggest use of EAA in my country is to synthesize medicines and their intermediates, mainly to synthesize γ-acetyl butyrolactone (an important intermediate of vitamin B) and 4-methyl-7-hydroxycoumarin (an anti-allergic drug). Intermediates), etc.; also used to prepare acetoacetyl o-chloroaniline (intermediate for the synthesis of 1,3,5-pyrazolone and Hansa yellow lake), acetoacetyl o-methylaniline (used for the synthesis of organic yellow lake) Intermediates of dyes such as dye packaging, synergistic pigment yellow); in addition, EAA is also used to produce pesticides such as pyrimidinophos-methyl, pyrimidinophos, etc. In foreign countries, it is also used as an unsaturated polyester co-accelerator and synthetic spices such as the preparation of linalool, ionone and macrocyclic spices. Ethyl acetoacetate is also used to synthesize 4-hydroxycoumarin, which is used to create the anticoagulant drug New Anticoagulant. Cyclization of ethyl acetoacetate with 1,3-bromochloropropane gives 2-methyl-3-ethoxycarbonyl-5,6-dihydropyran. This is an intermediate for the vasodilator pentoxifylline. Ethyl acetoacetate is also used in the synthesis of diclofenac sodium, amoxicillin, the selective coronary vasodilator Yantongxin and pyrimidine. The condensation of ethyl acetoacetate and aniline gives 4-hydroxyquinaldine. It is used in the production of disinfectant and antiseptic drug Clostridium. An intermediate of pyrazolone derivatives and dyes formed by the condensation of ethyl acetoacetate and phenylhydrazine. It also has important applications in pesticides, spices, photochemicals, polymerization catalysts, etc. Ethyl acetoacetate is also used as a food flavoring agent. Ethyl acetoacetate is also used as a solvent and as a reagent for the detection of thallium, calcium oxide, calcium hydroxide and copper.
4.Used as analytical reagents and solvents. Used in organic synthesis, dyes, medicine, plastics, paint and other industries.